Abstracts
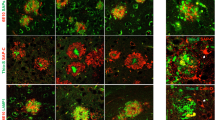
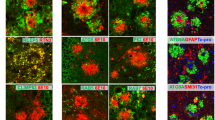
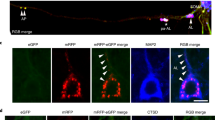
Progression of neuritic dystrophy is a histological hallmark of Alzheimer’s disease (AD) in addition to amyloid deposition and neurofibrillary tangle formation. Dystrophic neurites (DNs) are abnormal neurites, and are closely associated with amyloid deposits. To clarify the process of DN formation, we immunohistochemically investigated phosphorylated tau (AT8 and Ser396)-positive DNs and plaques in Tg2576 mice overexpressing human β-amyloid precursor protein (APP) with the Swedish type mutation (K670N/M671L). AT8-positive DNs were exclusively associated with the Congo red-positive plaques examined, and all Aβ1–40-positive plaques appeared to be associated with AT8-positive DNs, whereas there were no AT8-positive DNs with Aβ1–42-positive/Aβ1–40-negative plaques. Since we have previously shown that Aβ1–42-positive plaque precede Aβ1–40 deposition, the appearance of congophilic structures is also late. Quantitative analyses were performed on AT8-positive DNs that were associated with congophilic plaques in the cerebral cortex and hippocampus (more than 1,000 plaques). The number of congophilic plaques increased dramatically with age. The area of DNs in the cerebral cortex and hippocampus increased 120- and 60-fold from 11–13 to 20.5 months of age, respectively. Interestingly, the mean ratio of DN area to congophilic plaque area in every plaque was unchanged, approximately 10%, through the ages examined. The mean plaque size was stable with age in both the cortex and hippocampus. These data suggest that the formation of AT8-positive DNs is simultaneous with Congo red-positive plaque development, and that the event may be closely related in the pathological progression of AD.





Similar content being viewed by others
References
Armstrong RA (1998) β-amyloid plaques: stages in life history or independent origin? Dement Geriatr Cogn Disord 9:227–238
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82:239–259
Braak H, Braak E (1997) Staging of Alzheimer-related cortical destruction. Int Psychogeriatr 9:257–261
Carlson GA, Borchelt DR, Dake A, Turner S, Danielson V, Coffin JD, Eckman C, Meiners J, Nilsen SP, Younkin SG, Hsiao KK (1997) Genetic modification of the phenotypes produced by amyloid precursor protein overexpression in transgenic mice. Hum Mol Genet 6:1951–1959
Chapman PF, White GL, Jones MW, Cooper-Blacketer D, Marshall VJ, Irizarry M, Younkin L, Good MA, Bliss TV, Hyman BT, Younkin SG, Hsiao KK (1999) Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat Neurosci 2:271–276
Christie RH, Bacskai BJ, Zipfel WR, Williams RM, Kajdasz ST, Webb WW, Hyman BT (2001) Growth arrest of individual senile plaques in a model of Alzheimer’s disease observed by in vivo multiphoton microscopy. J Neurosci 21:858–864
Dickson DW, Farlo J, Davies P, Crystal H, Fuld P, Yen SH (1988) Alzheimer’s disease. A double-labeling immunohistochemical study of senile plaques. Am J Pathol 132:86–101
Fraser PE, Levesque L, McLachlan DR (1993) Biochemistry of Alzheimer’s disease amyloid plaques. Clin Biochem 26:339–349
Frautschy SA, Yang F, Irrizarry M, Hyman B, Saido TC, Hsiao K, Cole GM (1998) Microglial response to amyloid plaques in APPsw transgenic mice. Am J Pathol 152:307–317
Fukumoto H, Asami-Odaka A, Suzuki N, Iwatsubo T (1996) Association of Aβ 40-positive senile plaques with microglial cells in the brains of patients with Alzheimer’s disease and in non-demented aged individuals. Neurodegeneration 5:13–17
Fukumoto H, Asami-Odaka A, Suzuki N, Shimada H, Ihara Y, Iwatsubo T (1996) Amyloid β protein deposition in normal aging has the same characteristics as that in Alzheimer’s disease. Predominance of Aβ 42(43) and association of Aβ 40 with cored plaques. Am J Pathol 148:259–265
Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemens J, Donaldson T, Gillespie F, et al (1995) Alzheimer-type neuropathology in transgenic mice overexpressing V717F β-amyloid precursor protein. Nature 373:523–527
Greenberg SG, Davies P (1990) A preparation of Alzheimer paired helical filaments that displays distinct tau proteins by polyacrylamide gel electrophoresis. Proc Natl Acad Sci USA 87:5827–5831
Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G (1996) Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science 274:99–102
Ikeda K, Haga C, Kosaka K, Oyanagi S (1989) Senile plaque-like structures: observation of a probably unknown type of senile plaque by periodic-acid methenamine silver (PAM) electron microscopy. Acta Neuropathol 78:137–142
Irizarry MC, McNamara M, Fedorchak K, Hsiao K, Hyman BT (1997) APPSw transgenic mice develop age-related Aβ deposits and neuropil abnormalities, but no neuronal loss in CA1. J Neuropathol Exp Neurol 56:965–973
Iwatsubo T, Odaka A, Suzuki N, Mizusawa H, Nukina N, Ihara Y (1994) Visualization of Aβ 42(43) and Aβ 40 in senile plaques with end-specific Aβ monoclonals: evidence that an initially deposited species is Aβ 42(43). Neuron 13:45–53
Lorenzo A, Yankner BA (1994) β-amyloid neurotoxicity requires fibril formation and is inhibited by congo red. Proc Natl Acad Sci USA 91:12243–12247
Mann DM, Iwatsubo T, Ihara Y, Cairns NJ, Lantos PL, Bogdanovic N, Lannfelt L, Winblad B, Maat-Schieman ML, Rossor MN (1996) Predominant deposition of amyloid-β 42(43) in plaques in cases of Alzheimer’s disease and hereditary cerebral hemorrhage associated with mutations in the amyloid precursor protein gene. Am J Pathol 148:1257–1266
Masliah E, Sisk A, Mallory M, Mucke L, Schenk D, Games D (1996) Comparison of neurodegenerative pathology in transgenic mice overexpressing V717F β-amyloid precursor protein and Alzheimer’s disease. J Neurosci 16:5795–5811
McGeer PL, McGeer EG (1995) The inflammatory response system of brain: implications for therapy of Alzheimer and other neurodegenerative diseases. Brain Res Brain Res Rev 21:195–218
McKee AC, Kosik KS, Kowall NW (1991) Neuritic pathology and dementia in Alzheimer’s disease. Ann Neurol 30:156–165
Mehta PD, Dalton AJ, Mehta SP, Kim KS, Wisniewski HM, Aisen PS (1997) Plasma amyloid β1–42 levels are increased in Down’s syndrome but not in Alzheimer’s disease. Ann Neurol 42 (Suppl M29):400
Mehta PD, Dalton AJ, Mehta SP, Kim KS, Sersen EA, Wisniewski HM (1998) Increased plasma amyloid β protein 1–42 levels in Down syndrome. Neurosci Lett 241:13–16
Metsaars WP, Hauw JJ, Welsem ME van, Duyckaerts C (2003) A grading system of Alzheimer disease lesions in neocortical areas. Neurobiol Aging 24:563–572
Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, Belle G van, Berg L (1991) The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 41:479–486
Onorato M, Mulvihill P, Connolly J, Galloway P, Whitehouse P, Perry G (1989) Alteration of neuritic cytoarchitecture in Alzheimer disease. Prog Clin Biol Res 317:781–789
Pappolla MA, Chyan YJ, Omar RA, Hsiao K, Perry G, Smith MA, Bozner P (1998) Evidence of oxidative stress and in vivo neurotoxicity of β-amyloid in a transgenic mouse model of Alzheimer’s disease: a chronic oxidative paradigm for testing antioxidant therapies in vivo. Am J Pathol 152:871–877
Price DL, Tanzi RE, Borchelt DR, Sisodia SS (1998) Alzheimer’s disease: genetic studies and transgenic models. Annu Rev Genet 32:461–493
Selkoe DJ (1994) Alzheimer’s disease: a central role for amyloid. J Neuropathol Exp Neurol 53:438–447
Selkoe DJ (1998) The cell biology of β-amyloid precursor protein and presenilin in Alzheimer’s disease. Trends Cell Biol 8:447–453
Smith MA, Hirai K, Hsiao K, Pappolla MA, Harris PL, Siedlak SL, Tabaton M, Perry G (1998) Amyloid-β deposition in Alzheimer transgenic mice is associated with oxidative stress. J Neurochem 70:2212–2215
Sturchler-Pierrat C, Abramowski D, Duke M, Wiederhold KH, Mistl C, Rothacher S, Ledermann B, Burki K, Frey P, Paganetti PA, Waridel C, Calhoun ME, Jucker M, Probst A, Staufenbiel M, Sommer B (1997) Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc Natl Acad Sci USA 94:13287–13292
Styren SD, Hamilton RL, Styren GC, Klunk WE (2000) X-34, a fluorescent derivative of Congo red: a novel histochemical stain for Alzheimer’s disease pathology. J Histochem Cytochem 48:1223–1232
Su JH, Cummings BJ, Cotman CW (1998) Plaque biogenesis in brain aging and Alzheimer’s disease. II. Progressive transformation and developmental sequence of dystrophic neurites. Acta Neuropathol 96:463–471
Takeuchi A, Irizarry MC, Duff K, Saido TC, Hsiao Ashe K, Hasegawa M, Mann DM, Hyman BT, Iwatsubo T (2000) Age-related amyloid β deposition in transgenic mice overexpressing both Alzheimer mutant presenilin 1 and amyloid β precursor protein Swedish mutant is not associated with global neuronal loss. Am J Pathol 157:331–339
Terai K, Iwai A, Kawabata S, Sasamata M, Miyata K, Yamaguchi T (2001) Apolipoprotein E deposition and astrogliosis are associated with maturation of β-amyloid plaques in βAPPswe transgenic mouse: implications for the pathogenesis of Alzheimer’s disease. Brain Res 900:48–56
Terai K, Iwai A, Kawabata S, Tasaki Y, Watanabe T, Miyata K, Yamaguchi T (2001) β-amyloid deposits in transgenic mice expressing human β-amyloid precursor protein have the same characteristics as those in Alzheimer’s disease. Neuroscience 104:299–310
Tomidokoro Y, Ishiguro K, Harigaya Y, Matsubara E, Ikeda M, Park JM, Yasutake K, Kawarabayashi T, Okamoto K, Shoji M (2001) Aβ amyloidosis induces the initial stage of tau accumulation in APP(Sw) mice. Neurosci Lett 299:169–172
Westerman MA, Cooper-Blacketer D, Mariash A, Kotilinek L, Kawarabayashi T, Younkin LH, Carlson GA, Younkin SG, Ashe KH (2002) The relationship between Aβ and memory in the Tg2576 mouse model of Alzheimer’s disease. J Neurosci 22:1858–1867
Yilmazer-Hanke DM (1998) Pathogenesis of Alzheimer-related neuritic plaques: AT8 immunoreactive dystrophic neurites precede argyrophilic neurites in plaques of the entorhinal region, hippocampal formation, and amygdala. Clin Neuropathol 17:194–198
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Noda-Saita, K., Terai, K., Iwai, A. et al. Exclusive association and simultaneous appearance of congophilic plaques and AT8-positive dystrophic neurites in Tg2576 mice suggest a mechanism of senile plaque formation and progression of neuritic dystrophy in Alzheimer’s disease. Acta Neuropathol 108, 435–442 (2004). https://doi.org/10.1007/s00401-004-0907-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-004-0907-2