Abstract
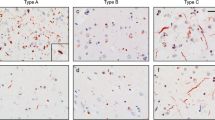
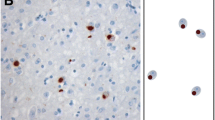
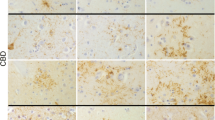
Ubiquitin-positive tau-negative inclusions were initially described in the rare form of frontotemporal dementia (FTD) associated with motor neuron disease. However, recent studies have indicated that these inclusions are also present in typical FTD, which is usually characterized by nonspecific histological changes. To examine the contribution of these inclusions to neuronal loss and to explore their relationship with disease duration, we performed a quantitative immunocytochemical analysis of 38 typical FTD cases. Relationships between neuron and ubiquitin inclusion densities as well as between duration of illness and neuropathological parameters was studied using linear regression in both univariate and multivariate models. Ubiquitin-positive tau-negative intracytoplasmic inclusions were present in 65.8% of cases in the dentate gyrus, 57.9% in temporal cortex and 31.6% in frontal cortex. The highest densities of ubiquitin-positive inclusions were consistently observed in the dentate gyrus, followed by the temporal and frontal cortex. There was no statistically significant relationship between neuron and ubiquitin-positive inclusion densities in any of the areas studied. In contrast, ubiquitin-positive inclusion densities in the dentate gyrus were negatively related to the duration of illness. Our data suggest that the development of ubiquitin-related pathology is the rule and not the exception in typical FTD, yet is not causally related to neuronal loss. They also reveal that the development of ubiquitin-positive inclusion densities in the dentate gyrus may be associated with a more aggressive form of the disease.


Similar content being viewed by others
References
Alves-Rodrigues A, Gregori L, Figueiredo-Pereira ME (1998) Ubiquitin, cellular inclusions and their role in neurodegeneration. Trends Neurosci 21:516–520
Bergmann M, Kuchelmeister K, Schmid KW, Kretzschmar HA, Schroder R (1996) Different variants of frontotemporal dementia: a neuropathological and immunohistochemical study. Acta Neuropathol 92:170–179
Bigio EH, Lipton AM, White CL 3rd, Dickson DW, Hirano A (2003) Frontotemporal and motor neurone degeneration with neurofilament inclusion bodies: additional evidence for overlap between FTD and ALS. Neuropathol Appl Neurobiol 29:239–253
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82:239–259
Brun A, Englund B, Gustafson L, Passant U, Mann D, Neary D, Snowden JS (1994) Clinical and neuropathological criteria for frontotemporal dementia. The Lund and Manchester Groups. J Neurol Neurosurg Psychiatry 57:416–418
Cairns NJ, Brannstrom T, Khan MN, Rossor MN, Lantos PL (2003) Neuronal loss in familial frontotemporal dementia with ubiquitin-positive, tau-negative inclusions. Exp Neurol 181:319–326
Deymeer F, Smith TW, DeGirolami U, Drachman DA (1989) Thalamic dementia and motor neuron disease. Neurology 39:58–61
Dickson DW (2001) Neuropathology of Pick’s disease. Neurology 56: S16–20
Ferrer I, Roig C, Espino A, Peiro G, Matias Guiu X (1991) Dementia of frontal lobe type and motor neuron disease. A Golgi study of the frontal cortex. J Neurol Neurosurg Psychiatry 54:932–934
Fischer DF, De Vos RA, Van Dijk R, De Vrij FM, Proper EA, Sonnemans MA, Verhage MC, Sluijs JA, Hobo B, Zouambia M, Steur EN, Kamphorst W, Hol EM, Van Leeuwen FW (2003) Disease-specific accumulation of mutant ubiquitin as a marker for proteasomal dysfunction in the brain. FASEB J 17:2014–2024
Forno LS, Langston JW, Herrick MK, Wilson JD, Murayama S (2002) Ubiquitin-positive neuronal and tau 2-positive glial inclusions in frontotemporal dementia of motor neuron type. Acta Neuropathol 103:599–606
Gomez-Isla T, Price JL, McKeel DW Jr, Morris JC, Growdon JH, Hyman BT (1996) Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J Neurosci 16:4491–4500
Hope AD, Silva R de, Fischer DF, Hol EM, Leeuwen FW van, Lees AJ (2003) Alzheimer’s associated variant ubiquitin causes inhibition of the 26S proteasome and chaperone expression. J Neurochem 86:394–404
Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL (1982) A new clinical scale for the staging of dementia. Br J Psychiatry 140:566–572
Ikeda K, Akiyama H, Arai T, Ueno H, Tsuchiya K, Kosaka K (2002) Morphometrical reappraisal of motor neuron system of Pick’s disease and amyotrophic lateral sclerosis with dementia. Acta Neuropathol 104:21–28
Ikemoto A, Hirano A, Akiguchi I, Kimura J (1997) Comparative study of ubiquitin immunoreactivity of hippocampal granular cells in amyotrophic lateral sclerosis with dementia, Guamanian amyotrophic lateral sclerosis and Guamanian parkinsonism-dementia complex. Acta Neuropathol 93:265–570
Iseki E, Li F, Odawara T, Hino H, Suzuki K, Kosaka K, Akiyama H, Ikeda K, Kato M (1998) Ubiquitin-immunohistochemical investigation of atypical Pick’s disease without Pick bodies. J Neurol Sci 159:194–201
Jackson M, Lennox G, Lowe J (1996) Motor neurone disease-inclusion dementia. Neurodegeneration 5:339–350
Kawashima T, Doh-ura K, Kikuchi H, Iwaki T (2001) Cognitive dysfunction in patients with amyotrophic lateral sclerosis is associated with spherical or crescent-shaped ubiquitinated intraneuronal inclusions in the parahippocampal gyrus and amygdala, but not in the neostriatum. Acta Neuropathol 102:467–472
Kertesz A, Kawarai T, Rogaeva E, St George-Hyslop P, Poorkaj P, Bird TD, Munoz DG (2000) Familial frontotemporal dementia with ubiquitin-positive, tau-negative inclusions. Neurology 54:818–827
Kinoshita A, Tomimoto H, Tachibana N, Suenaga T, Kawamata T, Kimura T, Akiguchi I, Kimura J (1996) A case of primary progressive aphasia with abnormally ubiquitinated neurites in the cerebral cortex. Acta Neuropathol 92:520–524
Kinoshita A, Tomimoto H, Suenaga T, Akiguchi I, Kimura J (1997) Ubiquitin-related cytoskeletal abnormality in frontotemporal dementia: immunohistochemical and immunoelectron microscope studies. Acta Neuropathol 94:67–72
Konagaya M, Sakai M, Matsuoka Y, Konagaya Y, Hashizume Y (1998) Upper motor neuron predominant degeneration with frontal and temporal lobe atrophy. Acta Neuropathol 96:532–536
Kövari E, Leuba G, Savioz A, Saini K, Anastasiu R, Miklossy J, Bouras C (2000) Familial frontotemporal dementia with ubiquitin inclusion bodies and without motor neuron disease. Acta Neuropathol 100:421–426
Kövari E, Gold G, Herrmann FR, Canuto A, Hof PR, Bouras C, Giannakopoulos P (2003) Lewy body densities in the entorhinal and anterior cingulate cortex predict cognitive deficits in Parkinson’s disease. Acta Neuropathol 106:83–88
Lang-Rollin I, Rideout H, Stefanis L (2003) Ubiquitinated inclusions and neuronal cell death. Histol Histopathol 18:509–517
Mackenzie IR, Feldman H (2004) Extrapyramidal features in patients with motor neuron disease and dementia; a clinicopathological correlative study. Acta Neuropathol 107:336–340
Mann DM, South PW, Snowden JS, Neary D (1993) Dementia of frontal lobe type: neuropathology and immunohistochemistry. J Neurol Neurosurg Psychiatry 56:605–614
Martin JA, Craft DK, Su JH, Kim RC, Cotman CW (2001) Astrocytes degenerate in frontotemporal dementia: possible relation to hypoperfusion. Neurobiol Aging 22:195–207
McKhann GM, Albert MS, Grossman M, Miller B, Dickson D, Trojanowski JQ (2001) Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick’s Disease. Arch Neurol 58:1803–1809
Mochizuki A, Komatsuzaki Y, Iwamoto H, Shoji S (2004) Frontotemporal dementia with ubiquitinated neuronal inclusions presenting with primary lateral sclerosis and parkinsonism: clinicopathological report of an autopsy case. Acta Neuropathol 107:337–380
Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, Boone K, Miller BL, Cummings J, Benson DF (1998) Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 51:1546–1554
Nichol KE, Kim R, Cotman CW (2001) Bcl-2 family protein behavior in frontotemporal dementia implies vascular involvement. Neurology 56:S35–40
Odawara T, Iseki E, Kanai A, Arai T, Katsuragi T, Hino H, Furukawa Y, Kato M, Yamamoto T, Kosaka K (2003) Clinicopathological study of two subtypes of Pick’s disease in Japan. Dement Geriatr Cogn Disord 15:19–25
Rosso SM, Kamphorst W, Graaf B de, Willemsen R, Ravid R, Niermeijer MF, Spillantini MG, Heutink P, Swieten JC van (2001) Familial frontotemporal dementia with ubiquitin-positive inclusions is linked to chromosome 17q21–22. Brain 124:1948–1957
Rosso SM, Donker Kaat L, Baks T, Joosse M, Koning I de, Pijnenburg Y, Jong D de, Dooijes D, Kamphorst W, Ravid R, Niermeijer MF, Verheij F, Kremer HP, Scheltens P, Duijn CM van, Heutink P, Swieten JC van (2003) Frontotemporal dementia in The Netherlands: patient characteristics and prevalence estimates from a population-based study. Brain 126:2016–2022
Sterio DC (1984) The unbiased estimation of number and sizes of arbitrary particles using the disector. J Microsc 134:127–136
Su JH, Nichol KE, Sitch T, Sheu P, Chubb C, Miller BL, Tomaselli KJ, Kim RC, Cotman CW (2000) DNA damage and activated caspase-3 expression in neurons and astrocytes: evidence for apoptosis in frontotemporal dementia. Exp Neurol 163:9–19
Talbot PR (1996) Frontal lobe dementia and motor neuron disease. J Neural Transm Suppl 47:125–132
Tolnay M, Probst A (1995) Frontal lobe degeneration: novel ubiquitin-immunoreactive neurites within frontotemporal cortex. Neuropathol Appl Neurobiol 21:492–497
Tolnay M, Probst A (2001) Frontotemporal lobar degeneration. An update on clinical, pathological and genetic findings. Gerontology 47:1–8
Toyoshima Y, Piao YS, Tan CF, Morita M, Tanaka M, Oyanagi K, Okamoto K, Takahashi H (2003) Pathological involvement of the motor neuron system and hippocampal formation in motor neuron disease-inclusion dementia. Acta Neuropathol 106:50–56
West MJ (1993) New stereological methods for counting neurons. Neurobiol Aging 14:275–285
West MJ, Coleman PD, Flood DG, Troncoso JC (1994) Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer’s disease. Lancet 344:769–772
Wightman G, Anderson VE, Martin J, Swash M, Anderton BH, Neary D, Mann D, Luthert P, Leigh PN (1992) Hippocampal and neocortical ubiquitin-immunoreactive inclusions in amyotrophic lateral sclerosis with dementia. Neurosci Lett 139:269–274
Woulfe J, Kertesz A, Munoz DG (2001) Frontotemporal dementia with ubiquitinated cytoplasmic and intranuclear inclusions. Acta Neuropathol 102:94–102
Yaguchi M, Okamoto K, Nakazato Y (2003) Frontotemporal dementia with cerebral intraneuronal ubiquitin-positive inclusions but lacking lower motor neuron involvement. Acta Neuropathol 105:81–85
Yang Y, Schmitt HP (2001) Frontotemporal dementia: evidence for impairment of ascending serotoninergic but not noradrenergic innervation. Immunocytochemical and quantitative study using a graph method. Acta Neuropathol 101:256–270
Zhukareva V, Vogelsberg-Ragaglia V, Van Deerlin VM, Bruce J, Shuck T, Grossman M, Clark CM, Arnold SE, Masliah E, Galasko D, Trojanowski JQ, Lee VM (2001) Loss of brain tau defines novel sporadic and familial tauopathies with frontotemporal dementia. Ann Neurol 49:165–175
Acknowledgements
We thank Mrs. P. Lovero and M. Surini-Demiri for the excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kövari, E., Gold, G., Giannakopoulos, P. et al. Cortical ubiquitin-positive inclusions in frontotemporal dementia without motor neuron disease: a quantitative immunocytochemical study. Acta Neuropathol 108, 207–212 (2004). https://doi.org/10.1007/s00401-004-0881-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-004-0881-8