Abstract
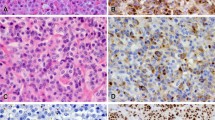
We present a unique case of a prolactin (PRL)-producing pituitary adenoma showing incomplete neuronal differentiation without ganglion cells. A 27-year-old man presented with nausea, headaches, and instability over the last 2 months. Clinical examination revealed obesity with no other endocrinological signs. His serum PRL levels were slightly elevated (38 ng/ml), whereas concentrations of the other adenohypophysial hormones were within normal range. Histology revealed an unusual pituitary adenoma containing many hypocellular areas with fibrillar appearance. The sizable tumor cells were polyhedral or elongated harboring an ovoid, vesicular nucleus with prominent nucleolus, lacking, however, the typical features of ganglion cells. By immunohistochemistry, many adenoma cells were positive for PRL. Immunostain for neurofilament protein revealed variable amounts of fibrils dispersed throughout the stroma, mostly in the hypocellular areas. In addition, neurofilament protein and chromogranin were strongly reactive in approximately 15% of the tumor cell population, whereas reactivity for synaptophysin was uniform throughout the tumor. These findings led to the conclusion that part of the tumor-cell population expressed a hybrid immunoprofile of adenoma–neuronal cell. Our case is the first PRL-producing pituitary adenoma showing incomplete neuronal differentiation lacking mature ganglion cells.







Similar content being viewed by others
References
Ahiman H, Wigander AL, Nilsson O (1989) Presence of nerve growth factor like immunoreactivity in carcinoid tumour cells and induction of a neuronal phenotype in long term culture. Int J Cancer 43:949–955
Asa SL, Scheithauer BW, Bilbao JM, Horvath E, Ryan N, Kovacs K, Randall RV, Laws ER Jr, Singer W, Linfoot JA, Thorner MO, Vale W (1984) A case for hypothalamic acromegaly: a clinicopathological study of six patients with hypothalamic gangliocytomas producing growth hormone-releasing factor. J Clin Endocrinol Metab 58:796–780
Bevan JS, Asa SL, Rossi ML, Esiri MM, Adams CB, Burke CW (1989) Intrasellar gangliocytoma containing gastrin and growth hormone-releasing hormone associated with a growth hormone-secreting pituitary adenoma. Clin Endocrionol (Oxf) 30:213–224
Dubois PM, El Anraoui A, Heretier AG (1997) Development and differentiation of pituitary cells. Microsc Res Tech 39:98–113
Franke WW, Grund C, Archatatter T (1986) Co-expression of cytokines and neurofilament proteins in a permanent cell line: Cultured rat PC12 cells combine neuronal and epithelial features. J Cell Biol 103:1933–1943
Geddes JF, Jansen GH, Robinson SFD, Gömöri E, Holton JL, Monson JP, Besser GM, Révész T (2000) Gangliocytomas of the pituitary. A heterogeneous group of lesions with differing histogenesis. Am J Surg Pathol 24:607–613
Horvath E, Kovacs K, Scheithauer BW, Lloyd RV, Smyth HS (1994) Pituitary adenoma with neuronal choristoma (PANCH): Composite lesion or lineage infidelity? Ultrastruct Pathol 18:565–574
Horvath E, Kovacs K, Tran E (2000) Ganglion cells in the posterior pituitary: result of ectopia or transdifferentiation? Acta Neuropathol 100:106–110
Kiyono H (1926) Die Histopathology der hypophyse. Virchows Arch A Pathol Anat Histopathol. 259:388–465
Lach B, Rippstein P, Benoit BG, Staines W (1996) Differentiating neuroblastoma of pituitary gland: neuroblastic transformation of epithelial adenoma cells. J Neurosurg 85:953–960
Li JY, Racadot O, Kujas M, Koyadri M, Peillon F, Racadot J (1989) Immunocytochemistry of four mixed pituitary adenomas and intrasellar gangliocytomas associated with different clinical syndromes: acromegaly, amenorrhea-galactorrhea, Cushing’s disease and isolated tumoral syndrome. Acta Neuropathol 77:320–328
Martinez-Campos A, Dannies PS (1986) A possible differentiation of anterior pituitary cells in collagen gels into neurons. Cell Tissue Res 244:21–26
McCann FV, Pettengill OS, Cole JJ, Russell JAG, Sorenson GD (1981) Calcium spike electrogenesis and other electrical activity in continuously cultured small cell carcinoma of the lung. Science 212:1155–1157
McCowen KC, Glickman JN, Black PMcL, Zervas NT, Lidov HGW, Garber JR (1999) Gangliocytoma masquerating as prolactinoma. J Neurosurg 91:490–495
Missale C, Boroni F, Sigala S, Buriani A, Fabris M, Leon A, Dal Toso R, Spano P (1996a) Nerve growth factor in the anterior pituitary: localization in mammotroph cells and cosecretion with prolactin by a dopamine-regulated mechanism. Proc Natl Acad Sci USA 93:4240–4245
Missale C, Losa M, Sigala S, Balsari A, Giovanelli M, Spano PF (1996b) Nerve growth factor controls proliferation and progression of human prolactinoma cell lines through an autocrine mechanism. Mol Endocrinol 10:272–285
Missale C, Fiorentini C, Finardi A, Spano P (1999) Growth factors in pituitary tumors. Pituitary 153–158
Nakamura M, Abe Y, Hatanaka H, Tsutsumi Y, Kijima H, Ueyama Y, Inoue H, Shimosato Y, Osamura RY (2003) Bronchial carcinoid tumor with gangliocytic- and paraganglionlike differentiation. Virchows Arch 442:183–185
Ogawa A, Sugihara S, Hasegawa M, Sasaki A, Nakazato Y, Kawada T, Ishiuchi S, Tamura M (1990) Intermediate filament expression in pituitary adenomas Virchows Arch B 58:341–349
Patterson JC, Childs GW (1994) Nerve growth factor and its receptor in the anterior pituitary. Endocrionol 135:1689–1696
Polak M, Scharfmann R, Seilheimer B, Eisenbarth G, Dressler D, Verma IM, Porter H (1993) Nerve growth factor induces neuron- like differentiation of an insulin- secreting pancreatic beta cell line. Proc Natl Acad Sci USA 90:5781–5785
Proesmans M, van Bael A, Andries M, Denef C (1997) Mitogenic effects of nerve growth on different cell types in reaggregate cell cultures of immature rat pituitary. Mol Cell Endocrinol 134:119–127
Puchner MJA, Lüdecke DK, Valdueza JM, Saeger W, Willig RP, Stalla GK, Odink RJ (1993) Cushing’s disease in a child caused by a corticotropin-releasing hormone-secreting intrasellar gangliocytoma associated with an adrenocorticotropic hormone-secreting pituitary adenoma. Neurosurgery 33:920–925
Puchner MJA, Lüdecke DK, Saeger W, Riedel M, Asa SL (1995) Gangliocytomas of the sellar region-a review. Exp Clin Endocrinol 103:129–149
Puchner MJΑ, Herrmann HD (1997) Intrasellar pituitary gangliocytoma-adenoma presenting with acromegaly: case report. Neurosurgery 40:611–614
Saeger W, Puchner MJA, Lüdecke DK (1994) combined sellar gangliocytoma and pituitary adenoma in acromegaly or Cushing’s disease. A report of three cases Virchows Arch 425:93–99
Schechter J, Windle JJ, Stauber C, Mellon PL (1992) Neural tissue within anterior pituitary tumors generated by oncogene expression in transgenic mice. Neuroendocrinol 56:300–311
Schechter J, Stauber C, Windle JJ, Mellon PL (1995) Basic fibroblastic growth factor: the neurotrophic factor influencing the ingrowth of neural tissue into the anterior pituitary of alpha-T7 transgenic mice? Neuroendocrinol 61:622–627
Scheithauer BW, Horvath E, Kovacs K, Lloyd RV, Stefaneanu L, Buchfelder M, Fahlbusch R, Werder K von, Lyons DF (1999) Prolactin-producing pituitary adenoma and carcinoma with neuronal components-a metaplastic lesion. Pituitary 1:197–205
Tamir H, Liu KP, Payette RF (1989) Human medullary thyroid carcinoma: characterization of the serotoninergic and neuronal properties of a neuroectodermally derived cell line. J Neurosci 9:1199–1212
Towfighi J, Salam MM, McLendon RE, Powers S, Page RB (1996) Ganglion-cell-containing tumors of the Pituitary Gland. Arch Pathol Lab Med 120:369–377
Vidal S, Horvath E, Bonert V, Shaninian H K, Kovacs K (2002) Neural transformation in pituitary corticotroph adenoma Acta Neuropathol 104:435–440
Acknowledgements
The authors are indebted to the National Hormone and Pituitary Program (NHPP), Torrance, California, USA, for providing the pituitary hormone antibodies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thodou, E., Kontogeorgos, G., Horvath, E. et al. Prolactin-producing pituitary adenoma with incomplete neuronal transformation: an intermediate adenoma–neuronal tumor. Acta Neuropathol 108, 115–120 (2004). https://doi.org/10.1007/s00401-004-0862-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-004-0862-y