Abstract
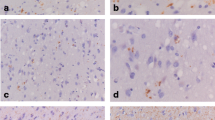
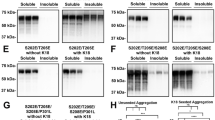
Abnormal tau hyperphosphorylation and deposition in Pick bodies is a major abnormality in Pick’s disease (PiD). This is associated with increased expression of the stress-activated protein kinase, p38 kinase, which has the capacity to phosphorylate tau in vitro. The present study has shown increased expression of phosphorylated p38 (p38-P), which does not cross-react with phospho-tau, in sarcosyl-insoluble fractions enriched in abnormal filaments, and hyperphosphorylated tau in the brain of two PiD cases obtained and processed with very short (less than 2 h) post-mortem delay. Immunohistochemical studies have shown p38-P co-localization in 90% of neurons with Pick bodies, whereas no positive cells are encountered in control brains processed in parallel. Moreover, p38-immunoprecipitated from sarcosyl-insoluble fractions in PiD brains is functionally active as it has the capacity to phosphorylate its specific substrate ATF-2. Combined biochemical, immunohistochemical and functional studies indicate that active p38 kinase is expressed in a very high percentage of Pick bodies, thus suggesting a critical role of this kinase in enhancing and perpetuating tau hyperphosphorylation in PiD.



Similar content being viewed by others
References
Arai T, Ikeda K, Akiyama H, Shikamoto Y, Tsuchiya K, Yagishita S, Beach T, Rogers J, Schwab C, McGeer I (2001) Distinct isoforms of tau aggregated in neurons and glial cells in brains of patients with Pick’s disease, corticobasal degeneration and progressive supranuclear palsy. Acta Neuropathol 101:167–173
Atzori C, Ghetti B, Piva R, Srinivasan AN, Zolo P, Delisle MB, Mirra SS, Migheli A (2001) Activation of JNK/p38 pathway occurs in diseases characterized by tau protein pathology and is related to tau phosphorylation but not to apoptosis. J Neuropathol Exp Neurol 60:1190–11197
Buée L, Delacourte A (1999) Comparative biochemistry of tau in progressive supranuclear palsy, corticobasal degeneration, FTDP-17 and Pick’s disease. Brain Pathol 9:681–693
Buée L, Bussière T, Buée-Cherrer V, Delacourte A, Hof PR (2000) Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Rev 33:95–130
Delacourte A, Robitaille Y, Sergeant N, Buée L, Hof PR, Wattez A, Laroche-Colette A, Matthie J, Chagnon P, Gauvreau D (1996) Specific pathological tau protein variants characterize Pick’s disease. J Neuropathol Exp Neurol 55:159–168
Dickson DW (1998) Pick’s disease: a modern approach. Brain Pathol 8:339–354
Ferrer I, Blanco R, Carmona M, Puig B (2001) Phosphorylated mitogen-activated protein kinase (MAPK/ERK-P), protein kinase of 38 kDa (p38-P), stress-activated protein kinase (SAPK/JNK-P), and calcium/calmodulin-dependent kinase II (CaM kinase II) are differentially expressed in tau deposits in neurons and glial cells in tauopathies. J Neural Transm 108:1397–1415
Ferrer I, Barrachina M, Tolnay M, Rey MJ, Vidal N, Carmona M, Blanco R, Puig B (2003) Phosphorylated protein kinases associated with neuronal and glial tau deposits in argyrophilic grain disease. Brain Pathol 13:62–78
Ferrer I, Friguls B, Dalfó E, Planas AM (2003) Early modifications in the expression of mitogen-activated protein kinase (MAPK/ERK), stress-activated kinases SAPK/JNK and p38, and their phosphorylated substrates following focal cerebral ischemia. Acta Neuropathol 105:425–437
Goedert M, Spillantini MG, Cairns NJ, Crowther RA (1992) Tau proteins of Alzheimer paired helical filaments: abnormal phosphorylation of all six brain isoforms. Neuron 8:159–168
Goedert M, Hasegawa M, Jakes R, Lawler S, Cuenda A, Cohen P (1997) Phosphorylation of microtubule-associated protein tau by stress-activated protein kinases. FEBS Lett 409:57–62
Hensley K, Floyd RA, Zheng NY, Nael R, Robinson KA, Nguyen X, Pye QN, Stewart CA, Geddes J, Marbersbery WR, Patel E, Johnson GV, Bing G (1999) p38 kinase is activated in the Alzheimer’s disease brain. J Neurochem 72:2053–2058
Lee MVY, Goedert M, Trojanowski JQ (2001) Neurodegenerative tauopathies. Annu Rev Neurosci 24:1121–1159
Markesbery WR, Carney JM (1999) Oxidative alterations in Alzheimer’s disease. Brain Pathol 9:133–146
Nunomura A, Perry G, Aliv G, Hirai K, Takeda A, Balraj EK, Jones PK, Ghanbari H, Wataya T, Shimohama S, Chiba S, Atwood C, Petersen RB, Smith MA (2001) Oxidative damage is the earliest event in Alzheimer’s disease. J Neuropathol Exp Neurol 60:759–767
Pei JJ, Braak E, Braak H, Grundke-Iqbal I, Iqbal K, Winblad W, Cowburn RF (2001) Localization of active forms of c-jun kinase (JNK) and p38 kinase in Alzheimer’s disease brains at different stages of neurofibrillary degeneration. J Alzheimer Dis 3:41–48
Reynolds CH, Nebreda AR, Gibbs GM, Utton MA, Anderton BH (1997) Reactivating kinase/p38 phosphorylates tau protein in vitro. J Neurochem 69:191–198
Reynolds CH, Utton MA, Gibb GM, Yates A, Anderton BH (1997) Stress-activated protein kinase/c-Jun N-terminal kinase phosphorylates tau protein. J Neurochem 68:1736–1744
Reynolds CH, Betts JC, Blackstock WP, Nebreda AR, Anderton BH (2000) Phosphorylation sites on tau identified by nanoelectrospray mass spectrometry: differences in vitro between the mitogen-activated protein kianase ERK2, c-Jun N-terminal kinase and p38, and glycogen synthase kinase-3beta. J Neurochem 74:1587–1595
Smith MA, Rottkamp CA, Nunomura A, Raina AK, Perry G (2000) Oxidative stress in Alzheimer’s disease. Biochim Biophys Acta 1502:132–144
Zhu X, Rottkamp CA, Boux H, Takeda A, Perry G, Smith MA (2000) Activation of p38 kinase links tau phosphorylation, oxidative stress, and cell cycle-related events in Alzheimer disease. J Neuropathol Exp Neurol 59:880–888
Zhu X, Raina AK, Rottkamp CA, Aliev G, Perry G, Boux H, Smith MA (2001) Activation and re-distribution of c-Jun N-terminal kinase/stress activated protein kinase in degenerating neurons in Alzheimer’s disease. J. Neurochem 76:435–441
Acknowledgements
This work was supported in part with the contract QLRI-CT-2000-00066, and grants SAF2001-4681-E, FIS PI020004, and FIS PI030032. We wish to thank Dr. B. Dèrijard, CNRS, Université de Nice for providing GST-ATF-2 used for immunoprecipitation, and T. Yohannan for editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Puig, B., Vinals, F. & Ferrer, I. Active stress kinase p38 enhances and perpetuates abnormal tau phosphorylation and deposition in Pick’s disease. Acta Neuropathol 107, 185–189 (2004). https://doi.org/10.1007/s00401-003-0793-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-003-0793-z