Abstract
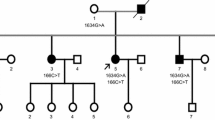
We clarified the clinical and pathological aspects of familial amyotrophic lateral sclerosis (FALS) with SOD1 H46R heterozygous mutation in the Miyakonojo Basin, a region in southern Japan where the prevalence of ALS is 11.4 per 105 of the population. We studied 17 patients, including one autopsy case, in three FALS families with the mutation. The average age at disease onset in the families was 44.3±8.7 years, and the mean disease duration was 12±7.6 years, with a range of 6 to 30 years. Ten of 17 patients were unable to walk by the mean age of 56.4±12.2 years. The initial symptom was muscle weakness in the distal leg muscle in all patients. The autopsy findings of one FALS patient showed atrophy of lateral and anterior funiculi, decreased numbers of anterior horn cells, preserved posterior funiculus and absence of neuronal inclusion bodies. Percentages of mutant SOD1 protein measured by mass spectrometry were 14% in erythrocytes, 43% in the spinal cord, 47% in the iliopsoas muscle and 60% in the diaphragm. In this study, we confirmed that FALS with SOD1 H46R mutation showed uniform initial symptoms and slow disease progression with intra-familial variation of disease severity and that inclusion body formation is not essential in FALS with this mutation.





Similar content being viewed by others
References
Abe K, Aoki M, Ikeda M, Watanabe M, Hirai S, Itoyama Y (1996) Clinical characteristics of familial amyotrophic lateral sclerosis with Cu/Zn superoxide dismutase gene mutation. J Neurol Sci 136:108–116
Aoki M, Ogasawara M, Matsubara Y, Narisawa K, Nakamura S, Itoyama Y, Abe K (1993) Mild ALS in Japan associated with novel SOD mutation. Nat Genet 5:323–324
Aoki M, Ogasawara M, Matsubara Y, Narisawa K, Nakamura S, Itoyama Y, Abe K (1994) Familial amyotrophic lateral sclerosis (ALS) in Japan associated With H46R mutation in Cu/Zn superoxide dismutase gene: a possible new subtype of familial ALS. J Neurol Sci 126:77–83
Bruijin LI, Houseweart MK, Kato S, Anderson KL, Anderson SD, Ohama E, Reaume AG, Scott RW, Cleveland DW (1998) Aggregation and motor neuron toxicity of an ALS-linked SOD1 mutant independent wild-type SOD1. Science 281:1851–1854
Cudkowicz ME, McKenna-Yasek D, Chen C, Hedley-Whyte ET, Brown RH Jr (1998) Limited corticospinal tract involvement in amyotrophic lateral sclerosis subjects with the A4 V mutation in the copper/zinc superoxide dismutase gene. Ann Neurol 43:703–710
Deng H-X, Hentati A, Tainer JA, Iqbal Z, Cayabyab A, Hung WY, Getzoff ED, Hu P, Herzfeldt B, Roos RP, Warner C, Deng G, Soiano E, Smyth C, Parge HE, Ahmed A, Roses AD, Hallewell RA, Pericak-Vance MA, Siddique T (1993) Amyotrophic lateral sclerosis and structural defects in Cu/Zn superoxide dismutase. Science 261:1047–1051
Fujita Y, Okamoto K, Sakurai A, Gonatas NK, Hirano A (2000) Fragmentation of the Golgi apparatus of the anterior horn cells in patients with familial amyotrophic lateral sclerosis with SOD1 mutations and posterior column involvement. J Neurol Sci 174:137–140
Haverkamp LJ, Appel V, Appel SH (1995) Natural history of amyotrophic lateral sclerosis in a database population. Validation of a scoring system and a model for survival prediction. Brain 118:707–719
Ince PG, Shaw PJ, Slade JY, Jones C, Hundgson P (1996) Familial amyotrophic lateral sclerosis with a mutation in exon 4 of the Cu/Zn superoxide dismutase gene: pathological and immunocytochemical changes. Acta Neuropathol 92:395–403
Julien J-P (2001) Amyotrophic lateral sclerosis: Unfolding the toxicity of the misfolded. Cell 104:581–591
Kadekawa J, Fujimura H, Ogawa Y, Hattori N, Kaido M, Nishimura T, Yoshikawa H, Shirahata N, Sakoda S, Yanagihara T (1997) A clinicopathological study of a patient with familial amyotrophic lateral sclerosis associated with a two base pair deletion in the coper/zinc superoxide dismutase (SOD1) gene. Acta Neuropathol 94:617–622
Kato S, Shimoda M, Watanabe Y, Nakashima K, Takahashi K, Ohama E (1996) Familial amyotrophic lateral sclerosis with a two base pair deletion in superoxide dismutase 1 gene: multisystem degeneration with intracytoplasmic hyaline inclusions in astrocytes. J Neuropathol Exp Neurol 55:1089–1101
Kohno S, Takahashi Y, Miyajima H, Serizawa M, Mizoguchi K (1999) A novel mutation (Cys6Gly) in the Cu/Zn superoxide dismutase gene associated with rapidly progressive familial amyotrophic lateral sclerosis. Neurosci Lett 276:135–137
Liu H, Zhu H, Eggers DK, Nersissian AM, Faull KF, Goto JJ, Ai J, Sanders-Loehr J, Gralla EB, Valentine JS (2000) Copper(2+) binding to the surface residue cysteine 111 of His46Arg human copper-zinc superoxide dismutase, a familial amyotrophic lateral sclerosis mutant. Biochemistry 39:8125–8132
Mourelatos Z, Hirano A, Rosenquist AC, Gonatas NK (1994) Fragmentation of golgi apparatus of motor neurons in amyotrophic lateral sclerosis (ALS). Clinical studies in ALS of Guam and experimental studies in deafferented neurons and in β,β’- iminodipropionitrile axonopathy. Am J Pathol 144:1288–1300
Nagai M, Aoki M, Miyoshi I, Kato M, Pasinelli P, Kasai N, Brown Jr RH, Itoyama Y, (2001) Rats expressing human cytosolic copper-zinc superoxide dismutase transgenes with amyotrophic lateral sclerosis: associated mutations develop motor neuron disease. J Neurosci 21:9246–9254
Nakanishi T, Kishikawa M, Miyazaki A, Shimizu A, Ogawa Y, Sakoda S, Ohi T, Shoji H (1998) Simple and defined method to detect the SOD1 mutants from patients with familial amyotrophic lateral sclerosis by mass spectrometry. J Neurosci Meth 81:41–44
Nakano R, Sato S, Inuzuka T, Sakimura K, Mishina M, Takahashi H, Ikuta F, Honma Y, Fujii J, Taniguchi N, Tsuji S (1994) A novel mutation in Cu/Zn superoxide dismutase gene in Japanese familial amyotrophic lateral sclerosis. Biochem Biophy Res Commun 200:695–703
Ohi T, Saita K, Takechi S, Nabesima K, Tshiro H, Shiomi K, Sugimoto S, Akematsu T, Nakayama T, Iwaki T, Matsukura S (2002) Clinical features and neuropathological findings of familial amyotrophic lateral sclerosis with a His46Arg mutation in Cu/Zn superoxide dismutase. J Neurol Sci 197:73–78
Okubo R, Arata H, Murakami T, Abe K, Arisato T, Nakagawa M (2001) Epidemiology of Motor Neuron Disease in the Miyakonojo Basin, Kyushu, Japan. In: Abe K (ed) Molecular mechanism and therapeutics of amyotrophic lateral sclerosis. Elsevier, Amsterdam, pp 35–40
Orrell RW, King AW, Hilton DA, Campbell MJ, Lane RJM, de Belleroche JS (1995) Familial amyotrophic lateral sclerosis with a point mutation of SOD-1: intrafamilial heterogeneity of disease duration associated with neurofibrillary tangles. J Neurol Neurosurg Psychiatry 59:266–270
Orrell RW, Habgood JJ, Malaspina A, Mitchell J, Greenwood J, Lane RJM, deBelleroche JS (1999) Clinical characteristics of SOD1 gene mutations in UK families with ALS. J Neurol Sci 169:56–60
Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, Oregan JP, Deng H-X, Rahmani Z, Krizus A, McKenna-Yasck D,Cayabyab A, Gaston SM, Berger R,Tanzi RE, Halperin JJ, Herzfeldt B, Van den Bergh R, Hung W-Y, Bird T, Deng G, Mulder DW, Smyth C, Laing NG, Soriano E, Pericak-Vance MA,Haines J, Rouleau GA, Gusella JS, Horritz HR, Brown RH (1993) Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362:59–62
Ross MA, Miller RG, Berchert L, Parry G, Barohn RJ, Armon C, Bryan WW, Petajan J, Stromatt S, Goodpasture J, McGuire D, the rhCNTF ALS Study Group (1998) Toward earlier diagnosis of amyotrophic lateral sclerosis; Revised criteria. Neurology 50:768–772
Rouleau GA, Clark AW, Rooke K, Pramatarova A, Krizus A, Suchowersky O, Julien JP, Figlewicz D (1996) SOD1 mutation is associated with accumulation of neurofilaments in amyotrophic lateral sclerosis. Ann Neurol 39:128–131
Shaw CE, Enayat ZE, Powell JF, Anderson VE, Radunovic A, al-Sarraj S, Leigh PN (1997) Familial amyotrophic lateral sclerosis. Molecular pathology of a patient with a SOD1 mutation. Neurology 49:1612–1616
Shibata N, Hirano A, Kobayashi M, Siddique T, Deng HX, Hung WX, Kato T, Asayama K (1996) Intense superoxide dismutase-1 immunoreactivity in intracytoplasmic hyaline inclusion of familial amyotrophic lateral sclerosis with posterior column involvement. J Neuropathol Exp Neurol 55:481–490
Takahashi H, Makifuchi T, Nakano R, Sato S, Inuzuka T, Sakimura K, Mishina M, Honma Y, Tsuji S, Ikuta F (1994) Familial amyotrophic lateral sclerosis with a mutation in the Cu/Zn superoxide dismutase gene. Acta Neuropathol 88:185–188
Yoshida S, Uebayashi Y, Kihira T, Kohmoto J, Wakayama I, Seiichiro T, Yase Y (1998) Epidemiology of motor neuron disease in the Kii Peninsula of Japan, 1989–1993: active or disappearing focus? J Neurol Sci 155:146–155
Wiederholt WC (1999) Neuroepidemiologic research initiatives on Guam: past and present. Neuroepidemiology 18:279–291
Acknowledgements
We express our appreciation to Dr. Jyoji Wakimoto (National Minamikyusyu Hospital, Kagoshima, Japan) for conducting the autopsy. We are grateful to Dr. Arlene R. Ng of Kagoshima University Faculty of Medicine for critical review. We also thank Ms. Yoko Tomita for her excellent technical assistance. This work was supported in part by a Research Grant (10B-4) for Nervous and Mental Disorders and a Research Grant (6-2A) for Central Nervous System Degenerative Disorders from the Ministry of Health, Labor and Welfare of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arisato, T., Okubo, R., Arata, H. et al. Clinical and pathological studies of familial amyotrophic lateral sclerosis (FALS) with SOD1 H46R mutation in large Japanese families. Acta Neuropathol 106, 561–568 (2003). https://doi.org/10.1007/s00401-003-0763-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-003-0763-5