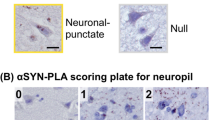
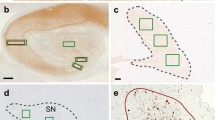
Abstract
To study the incidence and topographic distribution of α-synuclein-positive inclusions in Parkinson’s disease (PD), dementia with LB (DLB), and Alzheimer’s disease (AD), 206 brains of elderly patients, including 53 patients with clinical PD, 110 autopsy-proven AD cases, 22 with dementia with LB (DLB), 1 case with essential tremor, and 20 age-matched controls were investigated using α-synuclein immunohistochemistry. For technical reasons, the olfactory system was not studied. In all PD brains, α-synuclein-positive inclusions and neuronal losses were present in medullary and pontine nuclei, locus coeruleus, and substantia nigra, with additional lesions in amygdala (24%), allocortex (58%), cingulate area (34%), and isocortex (26.5%). All PD cases corresponded to pathology stage 4–6 suggested by Braak et al. (2003, Neurobiol Aging 24:197). In most cases of DLB, the distribution of α-synuclein pathology and neurodegeneration corresponded to stages 5 and 6 of PD pathology. The case with essential tremor and 48.2% of the AD cases showed no LB pathology; in the other AD brains α-synuclein-positive inclusions were seen in various brain areas. None of the controls showed LB pathology. Among 12 cases of incidental Lewy body disease (without clinical parkinsonian signs), 7 corresponded morphologically to PD stage 3 or 4. In further 6 AD cases, 2 with parkinsonian symptoms, considerable damage to locus coeruleus, substantia nigra, nucleus basalis and allocortex with preservation of the medullary nuclei was seen. The preliminary data largely confirm the Braak staging of brain pathology, although some of the clinical PD cases corresponded to stage 3 often considered as “preclinical”. In addition, some cases without demonstrable involvement of medullary nuclei showed extensive PD-like pathology in other brain areas, suggesting deviation from the proposed stereotypic expansion pattern and that incidental LB pathology may affect solely the locus coeruleus and substantia nigra. Striking similarity of LB pathology between DLB and PD suggests close morphological relationship between both disorders. Widespread LB lesions occurred in many sporadic AD cases without parkinsonian symptoms, the pathogenesis and clinical impact of which are unclear. The relationship between AD and PD with particular reference to synaptophysin-positive lesions needs further elucidation.







Similar content being viewed by others
References
American Psychiatric Association (1999) Diagnostic and statistical manual of mental disorders, 4th edn. American Psychiatric Association, Washington
Apaydin H, Ahlskog JE, Parisi JE, Boeve BF, Dickson DW (2002) Parkinson disease neuropathology: later-developing dementia and loss of the levodopa response. Arch Neurol 59:102–112
Arai Y, Yamazaki M, Mori O, Muramatsu H, Asano G, Katayama Y (2001) α-Synuclein-positive structures in cases with sporadic Alzheimer’s disease: morphology and its relationship to tau aggregation. Brain Res 888:287–296
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82:239–259
Braak H, Braak E, Yilmazer D, Vos RA de, Jansen EN, Bohl J (1996) Pattern of brain destruction in Parkinson’s and Alzheimer’s diseases. J Neural Transm 103:455–490
Braak H, Braak E, Yilmazer D, Vos RA de, Jansen EN, Bohl J, Jellinger K (1994) Amygdala pathology in Parkinson’s disease. Acta Neuropathol 88:493–500
Braak H, Del Tredici K, Bratzke H, Hamm-Clement J, Sandmann-Keil D, Rüb U (2002) Staging of the intracerebral inclusion body pathology associated with idiopathic Parkinson’s disease (preclinical and clinical stages). J Neurol 249(Suppl 3):III/1–5
Braak H, Del Tredici K, Rüb U, Vos RA de, Jansen Steur EN, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24:197–211
Braak H, Rüb U, Braak E (2000) Neuroanatomie des Morbus Parkinson. Veränderungen des neuronalen Zytoskeletts in nur wenigen für den Krankheitsprozess empfänglichen Nervenzelltypen führen zur progredienten Zerstörung umschriebener Bereiche des limbischen und des motorischen Systems. Nervenarzt 71:459–469
Braak H, Rüb U, Gai WP, Del Tredici K (2003) Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm. doi:10.1007/s00702-002-0808-2 (published online March 5, 2003)
Braak H, Rüb U, Sandmann-Keil D, Gai WP, Vos RA de, Jansen Steur EN, Arai K, Braak E (2000) Parkinson’s disease: affection of brain stem nuclei controlling premotor and motor neurons of the somatomotor system. Acta Neuropathol 99:489–495
Brown DF, Dababo MA, Bigio EH, Risser RC, Eagan KP, Hladik CL, White CL 3rd (1998) Neuropathologic evidence that the Lewy body variant of Alzheimer disease represents coexistence of Alzheimer disease and idiopathic Parkinson disease. J Neuropathol Exp Neurol 57:39–46
Damier P, Hirsch EC, Agid Y, Graybiel AM (1999) The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain 122:1437–1448
Del Tredici K, Rüb U, De Vos RA, Bohl JR, Braak H (2002) Where does parkinson disease pathology begin in the brain? J Neuropathol Exp Neurol 61:413–426
DeLucia MW, Cookson N, Dickson DW (2002) Synuclein-immunoreactive Lewy bodies are detected in the amygdala in less than 20% of Alzheimer’s disease (AD) cases. J Neuropathol Exp Neurol 61:454
Dickson DW (2001) α-Synuclein and the Lewy body disorders. Curr Opin Neurol 14:423–432
Duda JE, Giasson BI, Mabon ME, Lee VM, Trojanowski JQ (2002) Novel antibodies to synuclein show abundant striatal pathology in Lewy body diseases. Ann Neurol 52:205–210
Eadie MJ (1963) The pathology of certain medullary nuclei in parkinsonism. Brain 86:781–795
Fearnley JM, Lees AJ (1994) Pathology of Parkinson disease. In: Calne DB (ed) Neurodegenerative diseases. Saunders, Philadelphia, pp 545–554
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-Mental” state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Forno LS (1996) Neuropathology of Parkinson’s disease. J Neuropathol Exp Neurol 55:259–272
Gai WP, Blumbergs PC, Geffen LB, Blessing WW (1992) Age-related loss of dorsal vagal neurons in Parkinson’s disease. Neurology 42:2106–2111
Gelb DJ, Oliver E, Gilman S (1999) Diagnostic criteria for Parkinson disease. Arch Neurol 56:33–39
Gertz HJ, Xuereb J, Huppert F, Brayne C, McGee MA, Paykel E, Harrington C, Mukaetova-Ladinska E, Arendt T, Wischik CM (1998) Examination of the validity of the hierarchical model of neuropathological staging in normal aging and Alzheimer’s disease. Acta Neuropathol 95:154–158
Gibb WR, Lees AJ (1989) The significance of the Lewy body in the diagnosis of idiopathic Parkinson’s disease. Neuropathol Appl Neurobiol 15:27–44
Halliday GM, Li YW, Blumbergs PC, Joh TH, Cotton RG, Howe PR, Blessing WW, Geffen LB (1990) Neuropathology of immunohistochemically identified brain stem neurons in Parkinson’s disease. Ann Neurol 27:373–385
Hamilton RL (2000) Lewy bodies in Alzheimer’s disease: a neuropathological review of 145 cases using α-synuclein immunohistochemistry. Brain Pathol 10:378–384
Hansen L, Salmon D, Galasko D, et al (1990) The Lewy body variant of Alzheimer’s disease: a clinical and pathologic entity. Neurology 40:1–8
Harding AJ, Stimson E, Henderson JM, Halliday GM (2002) Clinical correlates of selective pathology in the amygdala of patients with Parkinson’s disease. Brain 125:2431–2445
Haroutunian V, Serby M, Purohit DP, Perl DP, Marin D, Lantz M, Mohs RC, Davis KL (2000) Contribution of Lewy body inclusions to dementia in patients with and without Alzheimer disease neuropathological conditions. Arch Neurol 57:1145–1150
Holstege G (1996) The somatic motor system. Prog Brain Res 107:9–26
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55:181–184
Hughes AJ, Daniel SE, Ben-Shlomo Y, Lees AJ (2002) The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain 125:861–870
Hurtig HI, Trojanowski JQ, Galvin J, Ewbank D, Schmidt ML, Lee VM, Clark CM, Glosser G, Stern MB, Gollomp SM, Arnold SE (2000) α-Synuclein cortical Lewy bodies correlate with dementia in Parkinson’s disease. Neurology 54:1916–1921
Ince PG, Perry EK, Morris CM (1998) Dementia with Lewy bodies. A distinct non-Alzheimer dementia syndrome? Brain Pathol 8:299–324
Ishizawa T, Mattila P, Davies P, Wang D, Dickson DW (2003) Colocalization of tau and α-synuclein epitopes in Lewy bodies. J Neuropathol Exp Neurol 62:389–397
Jellinger K (1991) Pathology of Parkinson’s disease. Changes other than the nigrostriatal pathway. Mol Chem Neuropathol 14:153–197
Jellinger KA (2001) The pathology of Parkinson’s disease. Adv Neurol 86:55–72
Jellinger KA, Bancher C (1998) Neuropathology of Alzheimer’s disease: a critical update. J Neural Transm Suppl 54:77–95
Jellinger KA, Mizuno Y (2003) Parkinson disease. In: Dickson DW, et al (eds) Neurodegeneration and dementias. ISN Press, Los Angeles (in press)
Jellinger KA, Seppi K, Wenning GK, Poewe W (2002) Impact of coexistent Alzheimer pathology on the natural history of Parkinson’s disease. J Neural Transm 109:329–339
Jellinger KA, Seppi K, Wenning GK (2003) Neuropathologic changes in Parkinson disease with late onset of dementia. (Letter) Arch Neurol 60:452–454
Kövari E, Gold G, Herrmenn FR, Canuto A, Hof PK, Bouras G, Giannakopoulos P (2003) Lewy body densities in the entorhinal and anterior cingulate cortex predict cognitive deficits in Parkinson disease. Acta Neuropathol 106:83–88
Lewy F (1913) Zur pathologischen Anatomie der Paralysis agitans. Dtsch Z Nervenheilkd 50:50–55
Lewy FH (1912) Paralysis agitans. Pathologische Anatomie. In: Lewandowsky M (ed) Handbuch der Neurologie. Springer, Berlin, pp 920–933
Lippa CF, Fujiwara H, Mann DM, Giasson B, Baba M, Schmidt ML, Nee LE, O’Connell B, Pollen DA, St George-Hyslop P, Ghetti B, Nochlin D, Bird TD, Cairns NJ, Lee VM, Iwatsubo T, Trojanowski JQ (1998) Lewy bodies contain altered α-synuclein in brains of many familial Alzheimer’s disease patients with mutations in presenilin and amyloid precursor protein genes. Am J Pathol 153:1365–1370
Lippa CF, Schmidt ML, Lee VM, Trojanowski JQ (1999) Antibodies to α-synuclein detect Lewy bodies in many Down’s syndrome brains with Alzheimer’s disease. Ann Neurol 45:353–357
Lowe J, Leigh PN (2002) Disorders of movement and system degenerations. In: Graham D, Lantos PL (eds) Greenfield’s neuropathology, 7th edn. Arnold, London, pp 325–430
Marui W, Iseki E, Nakai T, Miura S, Kato M, Ueda K, Kosaka K (2002) Progression and staging of Lewy pathology in brains from patients with dementia with Lewy bodies. J Neurol Sci 195:153–159
Mattila PM, Rinne JO, Helenius H, Dickson DW, Roytta M (2000) α-Synuclein-immunoreactive cortical Lewy bodies are associated with cognitive impairment in Parkinson’s disease. Acta Neuropathol 100:285–290
McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, Salmon DP, Lowe J, Mirra SS, Byrne EJ, Lennox G, Quinn NP, Edwardson JA, Ince PG, Bergeron C, Burns A, Miller BL, Lovestone S, Collerton D, Jansen EN, Ballard C, Vos RA de, Wilcock GK, Jellinger KA, Perry RH (1996) Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology 47:1113–1124
Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, Belle G van, Berg L (1991) The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 41:479–486
Nieuwenhuys R (1996) The greater limbic system, the emotional motor system and the brain. Prog Brain Res 107:551–580
Olszewski J, Baxter D (1982) Cytoarchitecture of the human brain stem, 2nd edn. Karger, Basel
Parkkinen L, Soininen H, Alafuzoff I (2003) Regional distribution of α-synuclein pathology in unimpaired aging and Alzheimer disease. J Neuropathol Exp Neurol 62:363–367
Pearce RK, Hawkes CH, Daniel SE (1995) The anterior olfactory nucleus in Parkinson’s disease. Mov Disord 10:283–287
Perl DP, Purohit DP, Haroutunian V (1997) Clinicopathologic correlations of the Alzheimer disease staging system introduced by Braak and Braak. J Neuropathol Exp Neurol 56:577
Perry RH, Irving D, Tomlinson BE (1990) Lewy body prevalence in the aging brain: relationship to neuropsychiatric disorders, Alzheimer-type pathology and catecholaminergic nuclei. J Neurol Sci 100:223–233
Schmidt ML, Martin JA, Lee VM, Trojanowski JQ (1996) Convergence of Lewy bodies and neurofibrillary tangles in amygdala neurons of Alzheimer’s disease and Lewy body disorders. Acta Neuropathol 91:475–481
Trembath Y, Rosenberg C, Ervin JF, Schmechel DE, Gaskell P, Pericak-Vance M, Vance J, Hulette CM (2003) Lewy body pathology is a frequent co-pathology in familial Alzheimer’s disease. Acta Neuropathol 105:484–488
Wakabayashi K, Toyoshima Y, Awamori K, Anezaki T, Yoshimoto M, Tsuji S, Takahashi H (1999) Restricted occurrence of Lewy bodies in the dorsal vagal nucleus in a patient with late-onset parkinsonism. J Neurol Sci 165:188–191
Mikolaenko L, Kwan CH, O’Brien R, Petnikov O, Troncoso JC (2003) Abstr. 6th International Conference AD|PD 2003; Seville, Spain, 8–12 May 2003; p 133
Acknowledgements
The author thanks the medical staff of Lainz Hospital, Wienerwald Geriatric Hospital, Otto Wagner Hospital, Vienna, and the Wagner-Jauregg Hospital, Linz, for clinical data, the pathologists of these hospital for autopsy reports and brain material, Mrs. V. Rappelsberger for excellent laboratory work, and E. Mitter-Ferstl, PhD, for secretarial and computer work. The study was supported by the Society for Support of Research in Experimental Neurology, Vienna, Austria.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s00401-003-0780-4
An erratum to this article is available at http://dx.doi.org/10.1007/s00401-003-0780-4.
Rights and permissions
About this article
Cite this article
Jellinger, K.A. α-Synuclein pathology in Parkinson’s and Alzheimer’s disease brain: incidence and topographic distribution—a pilot study. Acta Neuropathol 106, 191–202 (2003). https://doi.org/10.1007/s00401-003-0725-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-003-0725-y