Abstract
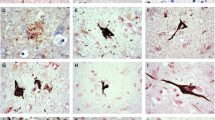
The microtubule-associated protein tau accumulates as cytoplasmic inclusions in Alzheimer's disease (AD), Pick's disease (PiD), progressive supranuclear palsy (PSP) and corticobasal degeneration (CBD). We investigated the immunoreactivity of tau-positive structures using a panel of antibodies to epitopes spanning the entire length of the tau molecule. In ethanol-fixed brain tissues, most antibodies to the microtubule-binding domain (MBD) required formic acid (FA) treatment to stain tau inclusions in PSP and CBD. This is in contrast with the intense labeling of neurofibrillary tangles in AD without FA treatment. Pick bodies (PiB) in PiD showed an intermediate pattern with respect to the immunoreactivity of the MBD because accumulated tau in PiB mostly lacks the insertion of exon 10, and the proportion of tau phosphorylated at Ser262 is smaller than in other abnormal tau structures. Such immunohistochemical profiles appeared to correlate with the occurrence of the smeared tau on immunoblot analysis of brain homogenate. The smeared tau was more abundant in AD and PiD than in PSP and CBD. Since the smeared tau was N-terminally truncated and was characteristic of advanced forms of modified tau, these findings suggest that tau accumulated in AD and PiD was processed more markedly than that in PSP and CBD. The MBD of tau may be masked in the presence of the intact N terminus and require FA treatment for antibody recognition in tissue sections. Advanced modification may expose the MBD in brain tissues of AD and PiD. It is suggested that the processing of abnormally accumulated tau characterizes the pathophysiology of each tauopathy.






Similar content being viewed by others
References
Arai T, Ikeda K, Akiyama H, Shikamoto Y, Tsuchiya K, Yagishita S, Beach T, Rogers J, Schwab C, McGeer PL (2001) Distinct isoforms of tau aggregated in neurons and glial cells in brains of patients with Pick's disease, corticobasal degeneration and progressive supranuclear palsy. Acta Neuropathol 101:167–173
Arai T, Ikeda K, Akiyama H, Tsuchiya K, Yagishita S, Takamatsu J (2001) Intracellular processing of aggregated tau differs between corticobasal degeneration and progressive supranuclear palsy. Neuroreport 12:935–938
Augustinack JC, Schneider A, Mandelkow E-A, Hyman BT (2001) Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer's disease. Acta Neuropathol 103:26–35
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82:239–259
Cammarata S, Mancardi G, Tabaton M (1990) Formic acid treatment exposes hidden neurofilament and tau epitopes in abnormal cytoskeletal filaments from patients with progressive supranuclear palsy and Alzheimer's disease. Neurosci Lett 115:351–355
Delacourte A, Sergeant N, Wattez A, Gauvreau D, Robitaille Y (1998) Vulnerable neuronal subsets in Alzheimer's and Pick's disease are distinguished by their τ isoform distribution and phosphorylation. Ann Neurol 43:193–204
Endoh R, Ogawara M, Iwatsubo T, Nakano I, Mori H (1993) Lack of the carboxyl terminal sequence of tau in ghost tangle of Alzheimer's disease. Brain Res 601:164–172
Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA (1989) Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer's disease. Neuron 3:519–526
Goedert M, Spillantini MG, Cairns NJ, Crowther RA (1992) Tau proteins of Alzheimer paired helical filaments: abnormal phosphorylation of all six brain isoforms. Neuron 8:159–168
Greenberg SG, Davies P, Schein JD, Binder LI (1992) Hydrofluoric acid-treated tau PHF proteins display the same biochemical properties as normal tau. J Biol Chem 267:564–569
Hof PR, Bouras C, Perl DP, Morrison JH (1994) Quantitative neuropathologic analysis of Pick's disease cases: cortical distribution of Pick bodies and coexistence with Alzheimer's disease. Acta Neuropahol 87:112–124
Ihara Y, Abraham C, Selkoe DJ (1983) Antibodies to paired helical filaments in Alzheimer's disease do not recognize normal brain proteins. Nature 304:727–730
Ikeda K, Akiyama H, Arai T, Tsuchiya K (2002) Pick-body-like inclusions in corticobasal degeneration differ from Pick bodies in Pick's disease. Acta Neuropahol 103:115–118
Ishiguro K, Sato K, Takamatsu M, Park J, Uchida T, Imahori K (1995) Analysis of phosphorylation of tau with antibodies specific for phosphorylation site. Neurosci Lett 202:81–84
Ishizawa K, Ksiezak-Reding H, Davies P, Delacourte A, Tiseo P, Yen SH, Dickson DW (2000) A double-labeling immunohistochemical study of tau exon 10 in Alzheimer's disease, progressive supranuclear palsy and Pick's disease. Acta Neuropahol 100:235–244
Jicha GA, Bowser R, Kazam IG, Davies P (1997) Alz-50 and MC-1, a new monoclonal antibody raised to paired helical filaments, recognizes conformational epitopes to recombinant tau. J Neurosci Res 48:128–132
Jicha GA, Lane E, Vincent I, Otvos L Jr, Hoffmann R, Davies P (1997) A conformation- and phosphorylation-dependent antibody recognizing the paired helical filaments of Alzheimer's disease. J Neurochem 69:2087–2095
Kametani F, Tanaka K, Ishii T, Ikeda S, Kennedy HE, Allsop D (1993) Secretory form of Alzheimer amyloid precursor protein 695 in human brain lacks β/A4 amyloid immunoreactivity. Biochem Biophys Res Commun 191:392–398
Kondo J, Honda T, Mori H, Hamada Y, Miura R, Ogawara M, Ihara Y (1988) The carboxyl third of tau is tightly bound to paired helical filaments. Neuron 1:827–834
Ksiezak-Reding H, Morgan K, Dickson DW (1994) Tau immunoreactivity and SDS solubility of two populations of paired helical filaments that differ in morphology. Brain Res 649:185–196
Mori H, Kondo J, Ihara Y (1987) Ubiquitin is a component of paired helical filaments in Alzheimer's disease. Science 235:1641–1644
Mori H, Hosoda K, Matsubara E, Nakamoto T, Furiya Y, Endoh R, Usami M, Shoji M, Maruyama S, Hirai S (1995) Tau in cerebrospinal fluids: establishment of the sandwich ELISA with antibody specific to the repeat sequence in tau. Neurosci Lett 186:181–183
Odawara T, Iseki E, Kanai A, Arai T, Katsuragi T, Hino H, Furukawa Y, Kato M, Yamamoto T, Kosaka K (2003) Clinicopathological study of two subtype of Pick's disease in Japan. Dement Geriatr Cogn Disord 15:19–25
Otvos L Jr, Feiner L, Lang E, Szendrei GI, Goedert M, Lee VM (1994) Monoclonal antibody PHF-1 recognizes tau protein phosphorylated at serine residues 396 and 404. J Neurosci Res 39:669–673
Probst A, Tolnay M, Langui D, Goedert M, Spillantini MG (1996) Pick's disease: hyperphosphorylated tau protein segregates to the somatoaxonal compartment. Acta Neuropahol 92:588–596
Sergeant N, Wattez A, Delacourte A (1999) Neurofibrillary degeneration in progressive supranuclear palsy and corticobasal degeneration: tau pathologies with exclusively "exon 10" isoforms. J Neurochem 72:1243–1249
Seubert P, Mawal-Dewan M, Barbour R, Jakes R, Goedert M, Johnson GVW, Literski JM, Schenk D, Lieberburg I, Trojanowski JQ, Lee VM-Y (1995) Detection of phosphorylated Ser262 in fetal tau, adult tau and paired helical filament-tau. J Biol Chem 270:18917–18922
Wang J-Z, Grundke-Iqbal I, Iqbal K (1996) Glycosylation of microtubule-associated protein tau: an abnormal posttranslational modification in Alzheimer's disease. Nat Med 2:871–875
Watanabe A, Takio K, Ihara Y (1999) Deamidation and isoaspartate formation in smeared tau in paired helical filaments. J Biol Chem 274:7368–7378
Wolozin GL, Pruchnicki A, Dickson DW, Davies P (1986) A neuronal antigen in the brain of Alzheimer's patients. Science 232:648–650
Zhukareva V, Mann D, Pikering-Brown S, Uryu K, Shunk T, Shah K, Grossman M, Miller BL, Hulette CM, Feinstein SC, Trojanowski JQ, Lee VMY (2002) Sporadic Pick's disease: a tauopathy characterized by a spectrum of pathological τ isoforms in gray and white matter. Ann Neurol 51:730–739
Acknowledgements
This study was supported by a grant-in-aid for encouragement of young scientists from the Ministry of Education, Science, Sports and Culture of Japan (12780576) and the Welfide Medicinal Research Foundation. We thank Dr. P. Davies (Albert Einstein College of Medicine, USA) for Alz-50, MC-1, TG3 and PHF-1, Dr. H. Mori (Osaka City University Medical School, Japan) for TF11, and Elan Pharmaceuticals for 12E8. We also thank Drs. T. Beach and J. Rogers for providing brain tissues.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arai, T., Ikeda, K., Akiyama, H. et al. Different immunoreactivities of the microtubule-binding region of tau and its molecular basis in brains from patients with Alzheimer's disease, Pick's disease, progressive supranuclear palsy and corticobasal degeneration. Acta Neuropathol 105, 489–498 (2003). https://doi.org/10.1007/s00401-003-0671-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-003-0671-8