Abstract
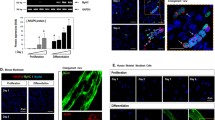
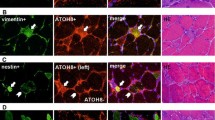
The molecular signaling pathways involved in regeneration after muscle damage have not been identified. In the present study, we tested the hypothesis that calcineurin, a calcium-regulated phosphatase recently implicated in the signaling of fiber-type conversion and muscle hypertrophy, is required to induce skeletal muscle remodeling. The amount of calcineurin and dephosphorylated nuclear factor of activated T cells c1 (NFATc1) proteins was markedly increased in the regenerating muscle of rats. The amount of calcineurin co-precipitating with NFATc1 and GATA-2, and NFATc1 co-precipitating with GATA-2 gradually increased in the tibialis anterior muscle after bupivacaine injection. Calcineurin protein was present in the proliferating satellite cells labeled with BrdU in the damaged muscle after 4 days. In contrast, calcineurin was not detected in the quiescent nonactivating satellite cells expressing Myf-5. At 4 days post injection, many macrophages detected in the damaged and regenerating area did not possess calcineurin protein. Calcineurin protein was abundant in many myoblasts and myotubes that expressed MyoD and myogenin at 4 and 6 days post injection. In the intact muscle, no immunoreactivity of calcineurin or BrdU was detected in the cell membrane, cytosol or the extracellular connective tissue. In mice, intraperitoneal injection of cyclosporin A, a potent inhibitor of calcineurin, induced extensive inflammation, marked fiber atrophy, the appearance of immature myotubes, and calcification in the regenerating muscle compared with phosphate-buffered saline-administered mice. Thus, calcineurin may have an important role in muscle regeneration in association with NFATc1 and GATA-2.







Similar content being viewed by others
References
Abbott KL, Friday BB, Thaloor D, Murphy TJ, Pavlath GK (1998) Activation and cellular localization of the cyclosporine A-sensitive transcription factor NF-AT in skeletal muscle cells. Mol Biol Cell 9:2905–2916
Beauchamp JR, Heslop L, Yu DSW, Tajbakhsh S, Kelly RG, Wernig A, Buckingham ME, Partridge TA, Zammit PS (2000) Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J Cell Biol 151:1221–1233
Bischoff R (1994) The satellite cell and muscle regeneration. In: Engel AG, Armstrong F (eds) Myology. McGraw-Hill, New York, pp 97–118
Calvo S, Venepally P, Cheng J, Buonanno A (1999) Fiber-type-specific transcription of the troponin I slow gene is regulated by multiple elements. Mol Cell Biol 19:515–525
Chakravarthy MV, Abraha TW, Schwartz RJ, Fiorotto ML, Booth FW (2000) Insulin- like growth factor-I extends in vitro replicative life span of skeletal muscle satellite cells by enhancing G1/S cell cycle progression via the activation of phosphatidylinositol 3'-kinase/Akt signaling pathway. J Biol Chem 275:35942–35952
Chin ER, Allen DG (1996) Changes in intracellular free Ca2+ concentration during constant 10 Hz stimulation of mouse single fibers. Physiologist 39:A75
Chin ER, Olson EN, Richardson JA, Yang Q, Humphries C, Shelton JM, Wu H, Zhu W, Bassel-Duby R, Williams RS (1998) A calcineurin-dependent transcriptional pathway controls skeletal muscle fiber type. Genes Dev 12:2499–2509
Coleman ME, Demayo F, Yin KC, Lee HM, Geske R, Montgomery C, Schwartz RJ (1995) Myogenic vector expression of insulin-like growth factor I stimulates muscle cell differentiation and myofiber hypertrophy in transgenic mice. J Biol Chem 270:12109–12116
Conboy IM, Manoli D, Mhaiskar V, Jones PP (1999) Calcineurin and vacuolar-type H+-ATPase modulate macrophage effector functions. Proc Natl Acad Sci USA 96:6324–6329
deFazio A, Leary JA, Hedley DW, Tattersal MH (1987) Immunohistochemical detection of proliferating cells in vivo. J Histochem Cytochem 35:571–577
Delling U, Tureckova J, Lim HW, De Windt LJ, Rotwein P, Molkentin JD (2000) A calcineurin-NFATc3-dependent pathway regulates skeletal muscle differentiation and slow myosin heavy-chain expression. Mol Cell Biol 20:6600–6611
DeVol DL, Rotwein P, Levis Sadow J, Npvakofski J, Bechtel PJ (1990) Activation of insulin-like growth factor gene expression during work-induced skeletal muscle growth. Am J Physiol 259:E89–E95
Dunn SE, Burns JL, Michel RN (1999) Calcineurin is required for skeletal muscle hypertrophy. J Biol Chem 274:21908–21912
Dunn SE, Chin ER, Michel RN (2000) Matching of calcineurin activity to upstream effectors is critical for skeletal muscle fiber growth. J Cell Biol 151:663–672
Engert JC, Berglund EB, Rosenthal N (1996) Proliferation precedes differentiation in IGF-I-stimulated myogenesis. J Cell Biol 135:431–440
Friday BB, Pavlath GK (2001) A calcineurin-and NFAT-dependent pathway regulates Myf-5 gene expression in skeletal muscle reserve cells. J Cell Sci 114:303–310
Friday BB, Horsley V, Pavlath GK (2000) Calcineurin activity is required for the initiation of skeletal muscle differentiation. J Cell Biol 149:657–665
Hawke TJ, Garry DJ (2001) Myogenic satellite cells: physiology to molecular biology. J Appl Physiol 91:534–551
Horsley V, Friday BB, Matteson S, Kegley KM, Gephart J, Pavlath GK (2001) Regulation of the growth of multinucleated muscle cells by an NFATC2-dependent pathway. J Cell Biol 153:329–338
Jennische E, Matejka GL (1992) IGF-I binding and IGF-I expression in regenerating skeletal muscle. Acta Physiol Scand 146:79–86
Kaye D, Pimental D, Prasad S, Maki T, Berger HJ, McNeil PL, Smith TW, Kelly RA (1996) Role of transiently altered sarcolemmal membrane permeability and basic fibroblast growth factor release in the hypertrophic response of adult rat ventricular myocytes to increased mechanical activity in vitro. J Clin Invest 97:281–291
Kurek JB, Nouri S, Kannourakis G, Murphy M, Lawrence A (1996) Leukemia inhibitory factor and interleukin-6 are produced by diseased and regenerating skeletal muscle. Muscle Nerve 19:1291–1301
McNeil PL, Steinhardt RA (1997) Loss, restoration, and maintenance of plasma membrane integrity. J Cell Biol 137:1–4
Megeney LA, Kablar B, Garrett K, Anderson JE, Rudnicki MA (1996) MyoD is required for myogenic stem cell function in adult skeletal muscle. Genes Dev 10:1173–1183
Mende U, Kagen A, Cohen A, Aramburu J, Schoen FJ, Neer EJ (1998) Transient cardiac expression of constitutively active Galphaq leads to hypertrophy and dilated cardiomyopathy by calcineurin-dependent and independent pathways. Proc Natl Acad Sci USA 95:13893–13898
Merly F, Lescaudron L, Rouaud T, Crossin F, Gardahaut MF (1999) Macrophages enhance muscle satellite cell proliferation and delay their differentiation. Muscle Nerve 22:724–732
Molkentin JD, Lu, J-R, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN (1998) A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 93:215–228
Musaró A, McCullagh KJA, Naya FJ, Olson EN, Rosenthal N (1999) IGF-I induces skeletal myocyte hypertrophy through calcineurin in association with GATA-2 and NF-ATc1. Nature 40:581–585
Musaró A, McCullagh K, Paul A, Houghton L, Dobrowolony G, Molinaro M, Barton ER, Sweeney HL, Rosenthal N (2001) Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat Genet 27:195–200
Naya FJ, Mercer B, Shelton J, Richadson JA, Williams RS, Olson EN (2000) Stimulation of slow skeletal muscle fiber gene expression by calcineurin in vivo. J Biol Chem 275:4545–4548
Pimorady-Esfahani A, Grounds MD, McMenamin PG (1997) Macrophages and dendritic cells in normal and regenerating murine skeletal muscle. Muscle Nerve 20:158–166
Sabourin LA, Girgis-Gabardo A, Seale P, Asakura A, Rudnicki MA (1999) Reduced differentiation potential of primary MyoD-/-cells derived from adult skeletal muscle. J Cell Biol 144:631–643
Sakuma K, Watanabe K, Totsuka T, Uramoto I, Sakamoto K, Sano M (1998) Differential adaptations of insulin-like growth factor, basic fibroblast growth factor and leukemia inhibitory factor in the plantaris muscle of rats by mechanical overloading: an immunohistochemical study. Acta Neuropathol 95:123–130
Sakuma K, Watanabe K, Sano M, Sakamoto K, Uramoto I, Totsuka T (1999) The adaptive response of MyoD family protein in the overloaded, regenerating and denervated rat muscles. Biochim Biophys Acta 1428:284–292
Sakuma K, Watanabe K, Sano M, Sakamoto K, Uramoto I, Totsuka T (2000) The adaptive response of transforming growth factor-β2 and βRII in the overloaded, regenerating and denervated muscles of rats. Acta Neuropathol 99:177–185
Sakuma K, Watanabe K, Sano M, Uramoto I, Totsuka T (2000) Differential adaptation of growth and differentiation factor 8/myostatin, fibroblast growth factor 6 and leukemia inhibitory factor in overloaded, regenerating and denervated rat muscles. Biochim Biophys Acta 1497:77–88
Sakuma K, Watanabe K, Sano M, Uramoto I, Nakano H, Li Y-J, Kaneda S, Sorimachi Y, Yoshimoto K, Yasuhara M, Totsuka T (2001) A possible role for BDNF, NT-4 and TrkB in the spinal cord and muscle of rat subjected to mechanical overload, bupivacaine injection and axotomy. Brain Res 907:1–19
Semsarian C, Wu M-J, Ju Y-K, Marciniec T, Yeoh T, Allen DG, Harvey RP, Graham RM (1999) Skeletal muscle hypertrophy is mediated by a Ca2+-dependent calcineurin signalling pathway. Nature 40:576–581
Sussman MA, Lim HW, Gude N, Taigen T, Olson EN, Robbins J, Colbert MC, Gualberto A, Wieczorek DF, Molkentin JD (1998) Prevention of cardiac hypertrophy in mice by calcineurin inhibition. Science 281:1690–1693
Tamaki T, Akatsuka A, Tokunaga M, Ishige K, Uchiyama S, Shiraishi T (1997) Morphological and biochemical evidence of muscle hyperplasia following weight-lifting exercise in rats. Am J Physiol 273:C246–C256
Torgan CE, Daniels MP (2001) Regulation of myosin heavy chain expression during rat skeletal muscle development in vitro. Mol Biol Cell 12:1499–1508
Westerblad H, Allen DG (1991) Changes in myoplasmic calcium concentration during fatigue in single mouse muscle fibers. J Gen Physiol 98:615–635
Acknowledgements
This work was supported by research grant nos. 10670775, 11780059, 12470108 and 13780029 from the Ministry of Education, Science, Sports and Culture of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sakuma, K., Nishikawa, J., Nakao, R. et al. Calcineurin is a potent regulator for skeletal muscle regeneration by association with NFATc1 and GATA-2. Acta Neuropathol 105, 271–280 (2003). https://doi.org/10.1007/s00401-002-0647-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-002-0647-0