Abstract
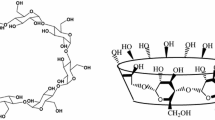
A method based on host-guest inclusion system was used to investigate the salt thickening mechanism of poly [acrylamide-co-N, N′-dimethyl (methylmethacryloyl ethyl) ammonium propane sulfonate-co-cetyl dimethyl allyl ammonium chloride] (PADC). The salt thickening behavior of PADC solution was studied by rotational viscometer, and its average hydrodynamic diameter (D h) was characterized by dynamic light scattering (DLS). The formed host (β-cyclodextrin)-guest (hydrophobic groups of PADC) inclusion system was confirmed by scanning electron microscopy (SEM). A method of characterizing the hydrophobic association strength and determining the hydrophobic association contribution rate (HACR) was introduced by the inclusion of β-cyclodextrin (β-CD). The results showed that the apparent viscosity and the D h of PADC solution increased with the increase of salt concentration. In addition, the types of salt also affected the thickening property of PADC solution. Moreover, the results of HACR indicated that the hydrophobic association strength of PADC solution was enhanced in the presence of salts, which was confirmed by fluorescent method. Thus, it could be concluded that the salt thickening of PADC solution was caused by the destruction of ionic bonds in betaine monomer and the enhancement of hydrophobic association strength.











Similar content being viewed by others
References
Shokrollahi A, Majidi SMJ, Ghazanfari MH (2013) Monitoring and characterizing the finger patterns developed by miscible displacement in fractured heavy oil systems Ind Eng Chem Res 52(31):10853–10863. doi:10.1021/ie4013908
Alquraishi AA, Alsewailem FD (2012) Xanthan and guar polymer solutions for water shut off in high salinity reservoirs Carbohydr Polym 88(3):859–863. doi:10.1016/j.carbpol.2012.01.022
Spildo K, Sæ EIØ (2015) Effect of charge distribution on the viscosity and viscoelastic properties of partially hydrolyzed polyacrylamide Energy Fuel 29(9):5609–5617. doi:10.1021/acs. energyfuels.5b01066
Zhao JZ, Jia H, Pu WF, Liao R (2011) Influences of fracture aperture on the water-shutoff performance of polyethyleneimine cross-linking partially hydrolyzed polyacrylamide gels in hydraulic fractured reservoirs Energy Fuel 25(6):2616–2624. doi:10.1021/ef200461m
Pu WF, Liu R, Wang KY, Li KX, Yan ZP, Li B, Zhao L (2015) Water soluble core-shell hyperbranched polymers for enhanced oil recovery Ind Eng Chem Res 54(3):798–807. doi:10.1021/ie5039693
Guo Y, Liu J, Zhang X, Feng R, Li H, Zhang J, Lv X, Luo P (2012) Solution property investigation of combination flooding systems consisting of gemini-non-ionic mixed surfactant and hydrophobically associating polyacrylamide for enhanced oil recovery Energy Fuel 26(4):2116–2123. doi:10.1021/ef202005p
Georgiev GS, Kamenska EB, Vassileva ED, Kamenova IP, Georgieva VT, Iliev SB, Ivanov IA (2006) Self-assembly, antipolyelectrolyte effect, and nonbiofouling properties of polyzwitterions Biomacromolecules 7(4):1329–1334. doi:10.1021/bm050938q
Johnson KM, Fevola MJ, Lochhead RY, Mccormick CL (2010) Hydrophobically modified acrylamide-based polybetaines. II. Interaction with surfactants in aqueous solution J Appl Polym Sci 92(1):658–671. doi:10.1002/app.13647
Ning J, Li G, Haraguchi K (2013) Synthesis of highly stretchable, mechanically tough, zwitterionic sulfobetaine nanocomposite gels with controlled thermosensitivities Macromolecules 46(13):5317–5328. doi:10.1021/ma4009059
Li Q, Bi QY, Zhou B, Wang XL (2012) Zwitterionic sulfobetaine-grafted poly(vinylidene fluoride) membrane surface with stably anti-protein-fouling performance via a two-step surface polymerization Appl Surf Sci 258(10):4707–4717. doi:10.1016/j.apsusc.2012.01.064
Zhang Q, Zhang S, Dai L, Chen X (2010) Novel zwitterionic poly(arylene ether sulfone)s as antifouling membrane material J Membrane Sci 349(1–2):217–224. doi:10.1016/j.memsci.2009.11.048
Kasák P, Kroneková Z, Krupa I, Lacík I (2011) Zwitterionic hydrogels crosslinked with novel zwitterionic crosslinkers: synthesis and characterization Polymer 52(14):3011–3020. doi:10.1016/j.polymer.2011.04.056
Longo GS, de la Cruz MO, Szleifer I (2013) pH-controlled nanoaggregation in amphiphilic polymer co-networks ACS Nano 7(3):2693–2704. doi:10.1021/nn400130c
Ye Z, Zhang X, Chen H, Han L, Jiang J, Song J, Yuan J (2015) Synthesis and evaluation of a class of sulfonic water soluble polymer with high content of nonionic surfmer units Colloid Polym Sci 293(8):2321–2330. doi:10.1007/s00396-015-3609-5
Liu KL, Zhang Z, Li J (2011) Supramolecular hydrogels based on cyclodextrin-polymer polypseudorotaxanes: materials design and hydrogel properties Soft Matter 7(24):11290–11297. doi:10.1039/c1sm06340e
Loftsson T, Masson M (2001) Cyclodextrins in topical drug formulations: theory and practice Int J Pharm 225(1–2):15–30. doi:10.1016/S0378-5173(01)00761-X
Roy A, Saha S, Roy MN (2016) Study to explore host-guest inclusion complexes of cyclodextrins with biologically active molecules in aqueous environment Fluid Phase Equilib 425:252–258. doi:10.1016/j.fluid.2016.06.013
Wang J, Qiu Z, Wang Y, Li L, Guo X, Pham DT, Lincoln SF, Prud'Homme RK (2016) Supramolecular polymer assembly in aqueous solution arising from cyclodextrin host-guest complexation Beilstein J Org Chem 12(1):50–72. doi:10.3762/bjoc.12.7
van de Manakker F, Vermonden T, van Nostrum CF, Hennink WE (2009) Cyclodextrin-based polymeric materials: synthesis, properties, and pharmaceutical/biomedical applications Biomacromolecules 10(12):3157–3175. doi:10.1021/bm901065f
Su Y, Li Q, Li S, Dan M, Huo F, Zhang W (2014) Doubly thermo-responsive brush-linear diblock copolymers and formation of core-shell-corona micelles Polymer 55(8):1955–1963. doi:10.1016/j.polymer.2014.02.060
Obeid R, Maltseva E, Thünemann AF, Tanaka F, Winnik FM (2009) Temperature response of self-assembled micelles of telechelic hydrophobically modified poly(2-alkyl-2-oxazoline)s in water Macromolecules 42(6):2204–2214. doi:10.1021/ma802592f
Shaikh S, Asrof Ali SK, Hamad EZ, Al-Nafaa M, Al-Jarallah A, Abu-Sharkh B (1998) Synthesis, characterization, and solution properties of hydrophobically modified poly(vinyl alcohol) J Appl Polym Sci 70(12):2499–2506. doi:10.1002/(SICI)1097-4628(19981219)70:12<2499::AID-APP23>3.0.CO;2-9
Zhou C, Yang W, Yu Z, Zhou W, Xia Y, Han Z, Wu Q (2011) Synthesis and solution properties of novel comb-shaped acrylamide copolymers Polym Bull 66(3):407–417. doi:10.1007/s00289-010-0360-4
Liaw DJ, Huang CC, Sang HC, Kang ET (2001) Aqueous solution and photophysical properties of cationic poly(trimethyl methacrylamidophenyl ammonium methylsulfate) and zwitterionic poly( N, N -dimethylmethacrylamidophenyl ammonium propane sultone) Polymer 42(1):209–216. doi:10.1016/S0032-3861(00)00310-4
Rushing TS, Hester RD (2004) Semi-empirical model for polyelectrolyte intrinsic viscosity as a function of solution ionic strength and polymer molecular weight Polymer 45(19):6587–6594. doi:10.1016/j.polymer.2004.07.029
Ye T, Song Y, Zheng Q (2016) Salt response and rheological behavior of acrylamide-sulfobetaine copolymer Colloid Polym Sci 294(2):389–397. doi:10.1007/s00396-015-3790-6
Shannon RD (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides Acta Crystallogr Sect A Found Crystallogr 32(5):751–767
Che Y, Tan Y, Cao J, Xu G (2010) Aggregation behavior of copolymer containing sulfobetaine structure in aqueous solution J Macromol Sci B 49(4):695–710. doi:10.1080/00222341003598281
Sarsenbekuly B, Kang W, Fan H, Yang H, Dai C, Zhao B, Aidarova SB (2017) Study of salt tolerance and temperature resistance of a hydrophobically modified polyacrylamide based novel functional polymer for EOR Colloid Surf A 514:91–97. doi:10.1016/j.colsurfa.2016.10.051
Rashidi M, Blokhus AM, Skauge A (2010) Viscosity study of salt tolerant polymers J Appl Polym Sci 117(3):1551–1557. doi:10.1002/app.32011
Hou C, Wang CG, Ying L, Cai HS (2003) Effect of inorganic salts on viscosity of acrylonitrile- N -vinylpyrrolidone copolymer solutions J Appl Polym Sci 89(13):3492–3495. doi:10.1002/app.12501
Li L, Guo X, Fu L, Prud'Homme RK, Lincoln SF (2008) Complexation behavior of alpha-, beta-, and gamma-cyclodextrin in modulating and constructing polymer networks Langmuir 24(15):8290–8296. doi:10.1021/la800859w
Sinquin A, Hubert P, Dellacherie E (1993) Amphiphilic derivatives of alginate: evidence for intra- and intermolecular hydrophobic associations in aqueous solution Langmuir 9(12):3334–3337. doi:10.1021/la00036a002
Kang W, Ji Y, Xu B, Hu L, Meng L, Fan H, Bai B (2014) Research on association between multi-sticker amphiphilic polymer and water-soluble beta-cyclodextrin polymer Colloid Polym Sci 292(4):895–903. doi:10.1007/s00396-013-3118-3
Basak P, Nisha CK, Manorama SV, Maiti S, Jayachandran KN (2003) Probing the association behavior of poly(ethylene glycol)-based amphiphilic comb-like polymer in NaCl solution J Colloid Interface Sci 262(2):560–565. doi:10.1016/S0021-9797(03)00119-X
Tian Q, Zhao X, Tang X, Zhang Y (2003) Hydrophobic association and temperature and pH sensitivity of hydrophobically modified poly(N-isopropylacrylamide/acrylic acid) gels J Appl Polym Sci 87(14):2406–2413. doi:10.1002/app.12122
Lüdemann S, Abseher R, Schreiber H, Steinhauser O (1997) The temperature-dependence of hydrophobic association in water. Pair versus bulk hydrophobic interactions J Am Chem Soc 119(18):4206–4213. doi:10.1021/ja953439d
Acknowledgments
The work was supported by the Fundamental Research Funds for the Central Universities (16CX06032A, 15CX08003A), the National Natural Science Foundation of China (21273286), and the Qingdao Postdoctoral Application Research Project (2016222).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 467 kb)
Rights and permissions
About this article
Cite this article
Zhu, Z., Kang, W., Yang, H. et al. Study on salt thickening mechanism of the amphiphilic polymer with betaine zwitterionic group by β-cyclodextrin inclusion method. Colloid Polym Sci 295, 1887–1895 (2017). https://doi.org/10.1007/s00396-017-4169-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-017-4169-7