Abstract
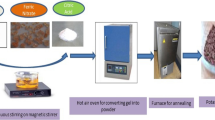
Using a sol–gel method, silica-coated magnetite (Fe3O4 @SiO2) core-shell nanoparticles were fabricated following a two-step process. In the first step, the Fe3O4 nanoparticles were prepared via a solvothermal method. In the second step, the Fe3O4 nanoparticles were coated with SiO2 formed through the hydrolyzation of tetraethyl orthosilicate. The structure and properties of the core-shell Fe3O4 @SiO2 nanoparticles were characterized and the results showed that Fe3O4 @SiO2 nanoparticles are a soft magnetic material. A magnetorheological (MR) suspension was prepared based on the synthesized Fe3O4 @SiO2 nanoparticles dispersed in silicone oil and measured using a rotational rheometer at various magnetic field strengths. Using a rotational rheometer, the MR properties of the Fe3O4 @SiO2 in silicone oil, including shear stress, shear viscosity, and yield stress were examined under an applied magnetic field.










Similar content being viewed by others
References
Liu ZL, Wang HB, Lu QH, Du GH, Peng L, Du YQ, Zhang SM, Yao KL (2004) Synthesis and characterization of ultrafine well-dispersed magnetic nanoparticles. J Magn Magn Mater 283:258–262
Kim DK, Mikhaylova M, Wang F-H, Kehr J, Bjelke B, Zhang Y, Tsakalakos T, Muhammed M (2003) Starch-coated superparamagnetic nanoparticles as MR contrast agents. Chem Mater 15:4343–4351
Kobayashi Y, Horie M, Konno M, Rodriguez-Gonzalez B, Liz-Marzan LM (2003) Preparation and properties of silica-coated cobalt nanoparticles. J Phys Chem B 107:7420–7425
Sun SH, Murray CB, Weller D, Folks L, Moser A (2000) Monodisperse FePt nanoparticles and ferromagnetic FePt nanocrystal superlattices. Science 287:1989–1992
Du GH, Liu ZL, Xia X, Chu Q, Zhang SM (2006) Characterization and application of Fe3O4/SiO2 nanocomposites. J Sol–gel Sci Technol 39:285–291
Azurdia JA, Marchal J, Shea P, Sun HP, Pan XQ, Laine RM (2006) Liquid-feed flame spray pyrolysis as a method of producing mixed-metal oxide nanopowders of potential interest as catalytic materials. Nanopowders along the NiO-Al2O3 tie line including (NiO)0.22(Al2O3)0.78, a new inverse spinel composition. Chem Mater 18:2458–2458
Sivakov V, Petersen C, Daniel C, Shen H, Mucklich F, Mathur S (2005) Laser induced local and periodic phase transformations in iron oxide thin films obtained by chemical vapour deposition. Appl Surf Sci 247:513–517
Sakabe Y, Yamashita Y, Yamamoto H (2005) Dielectric properties of nano-crystalline BaTiO3 synthesized by micro-emulsion method. J Eur Ceram Soc 25:2739–2742
Fujii T, Matsusue I, Nakatsuka D, Nakanishi M, Takada J (2011) Synthesis and anomalous magnetic properties of LaFeO3 nanoparticles by hot soap method. Mater Chem Phys 129:805–809
Shi D, Hu GH, Li RKY (2006) Concept of nano-reactor for the control of the selectivity of the free radical grafting of maleic anhydride onto polypropylene in the melt. Chem Eng Sci 61:3780–3784
Cao JQ, Wang YX, Yu JF, Xia JY, Zhang CF, Yin DZ, Hafeli UO (2004) Preparation and radiolabeling of surface-modified magnetic nanoparticles with rhenium-188 for magnetic targeted radiotherapy. J Magn Magn Mater 277:165–174
Sahin M, Ayranci K, Kosun E, Ayranci E (2009) Density, sound velocity and viscosity properties of aqueous sodium metatungstate solutions and an application of these solutions in heavy mineral separations. Chem Geol 264:96–100
Alexiou C, Schmidt A, Klein R, Hulin P, Bergemann C, Arnold W (2002) Magnetic drug targeting: biodistribution and dependency on magnetic field strength. J Magn Magn Mater 252:363–366
Pai DM, Springett BE (1993) Physics of electrophotography. Rev Mod Phys 65:163–211
Jordan A, Scholz R, Wust P, Fahling H, Felix R (1999) Magnetic fluid hyperthermia (MFH): cancer treatment with AC magnetic field induced excitation of biocompatible superparamagnetic nanoparticles. J Magn Magn Mater 201:413–419
Shen LF, Laibinis PE, Hatton TA (1999) Bilayer surfactant stabilized magnetic fluids: synthesis and interactions at interfaces. Langmuir 15:447–453
Jordan A, Scholz R, Wust P, Schirra H, Schiestel T, Schmidt H, Felix R (1999) Endocytosis of dextran and silan-coated magnetite nanoparticles and the effect of intracellular hyperthermia on human mammary carcinoma cells in vitro. J Magn Magn Mater 194:185–196
Li Q, Xuan YM, Wang J (2005) Experimental investigations on transport properties of magnetic fluids. Exp Thermal Fluid Sci 30:109–116
Hong RY, Li JH, Zhang SZ, Li HZ, Zheng Y, Ding JM, Wei DG (2009) Preparation and characterization of silica-coated Fe3O4 nanoparticles used as precursor of ferrofluids. Appl Surf Sci 255:3485–3492
Bocanegra-Diaz A, Mohallem NDS, Novak MA, Sinisterra RD (2004) Preparation of ferrofluid from cyclodextrin and magnetite. J Magn Magn Mater 272:2395–2397
Li ZW, Zhu YF (2003) Surface-modification of SiO2 nanoparticles with oleic acid. Appl Surf Sci 211:315–320
Goguet A, Schweich D, Candy JP (2003) Preparation of a Pt/SiO2 catalyst II. Temperature-programmed decomposition of the adsorbed platinum tetrammine hydroxide complex under flowing hydrogen, oxygen, and argon. J Catal 220:280–290
Li L, Zhang LY, Yao X (2004) Preparation and characterization of thick porous SiO2 film for multilayer pyroelectric thin film IR detector. Ceram Int 30:1843–1846
Nontasorn P, Chavadej S, Rangsunvigit P, O’Haver JH, Chaisirimahamorakot S, Na-Ranong N (2005) Admicellar polymerization modified silica via a continuous stirred-tank reactor system: Comparative properties of rubber compounding. Chem Eng J 108:213–218
Kamimura K, Kobayashi D, Okada S, Mizuguchi T, Ryu E, Hayashibe R, Nagaune F, Onuma Y (2001) Preparation and characterization of SiO2/6H-SiC metal-insulator-semiconductor structure using TEOS as source material. Appl Surf Sci 184:346–349
Santiago-Quinones DI, Acevedo A, Rinaldi C (2009) Magnetic and magnetorheological characterization of a polymer liquid crystal ferronematic. J Appl Phys 105
Bica I (2009) Influence of the transverse magnetic field intensity upon the electric resistance of the magnetorheological elastomer containing graphite microparticles. Mater Lett 63:2230–2232
Mrlik M, Ilcikova M, Pavlinek V, Mosnacek J, Peer P, Filip P (2013) Improved thermooxidation and sedimentation stability of covalently-coated carbonyl iron particles with cholesteryl groups and their influence on magnetorheology. J Colloid Interf Sci 396:146–151
Pfeil KV, Graham MD, Klingenberg DJ, Morris JF (2003) Structure evolution in electrorheological and magnetorheological suspensions from a continuum perspective. J Appl Phys 93:5769–5779
Bica I, Anitas EM, Averis LME, Bunoiu M (2015) Magnetodielectric effects in composite materials based on paraffin, carbonyl iron and graphene. J Ind Eng Chem 21:1323–1327
Bica I, Anitas EM (2015) Averis LME (2015) Tensions and deformations in composites based on polyurethane elastomer and magnetorheological suspension: effects of the magnetic field. J Ind Eng Chem 28:86–90
Carlson JD (2002) What makes a good MR fluid? J Intel Mater Syst Struct 13:431–435
Hu W, Cook E, Wereley NM (2007) Energy absorber using a magnetorheological bypass valve filled with ferromagnetic beads. IEEE Trans Magn 43:2695–2697
Li WH, Du H, Guo NQ (2004) Dynamic behavior of MR suspensions at moderate flux densities. Mater Sci Eng A-Struct 371:9–15
Choi HJ, Jang IB, Lee JY, Pich A, Bhattacharya S, Adler HJ (2005) Magnetorheology of synthesized core-shell structured nanoparticle. IEEE Trans Magn 41:3448–3450
Liu YD, Choi HJ, Choi SB (2012) Controllable fabrication of silica encapsulated soft magnetic microspheres with enhanced oxidation-resistance and their rheology under magnetic field. Colloids Surf A 403:133–138
Chae HS, Piao SH, Choi HJ (2015) Fabrication of spherical Fe3O4 particles with a solvothermal method and their magnetorheological characteristics. J Ind Eng Chem 29:129–133
Stober AFW (1968) Controlled growth of monodisperse silica spheres in the micron size range. J Colloid Interf Sci 26:62–69
Singh K, Ohlan A, Pham VH, Balasubramaniyan R, Varshney S, Jang J, Hur SH, Choi WM, Kumar M, Dhawan SK, Kong BS, Chung JS (2013) Nanostructured graphene/Fe3O4 incorporated polyaniline as a high performance shield against electromagnetic pollution. Nanoscale 5:2411–2420
Nallathambi G, Ramachandran T, Rajendran V, Palanivelu R (2011) Effect of silica nanoparticles and BTCA on properties of cotton fabrics. Mater Res-Ibero-Am J 14:552–559
Balau O, Bica D, Koneracka M, Kopcansky P, Susan-Resiga D, Vekas L (2002) Rheological and magnetorheological behaviour of some magnetic fluids on polar and nonpolar carrier liquids. Int J Mod Phys B 16:2765–2771
Ahmadkhanlou F, Mahboob M, Bechtel S, Washington G (2010) An improved model for magnetorheological fluid-based actuators and sensors. J Intel Mater Syst Struct 21:3–18
Fang FF, Choi HJ (2010) Fabrication of multiwalled carbon nanotube-wrapped magnetic carbonyl iron microspheres and their magnetorheology. Colloid Polym Sci 288:79–84
Park BJ, Hong MK, Choi HJ (2009) Atom transfer radical polymerized PMMA/magnetite nanocomposites and their magnetorheology. Colloid Polym Sci 287:501–504
Fang FF, Choi HJ, Seo Y (2010) Sequential coating of magnetic carbonyl iron particles with polystyrene and multiwalled carbon nanotubes and its effect on their magnetorheology. ACS Appl Mater Interfaces 2:54–60
Liu YD, Fang FF, Choi HJ (2011) Core-shell-structured silica-coated magnetic carbonyl iron microbead and its magnetorheology with anti-acidic characteristics. Colloid Polym Sci 289:1295–1298
Huang H, You B, Zhou S, Wu L (2007) Rheological behavior of aqueous organosilicone resin emulsion stabilized by colloidal nanosilica particles. J Colloid Interface Sci 310:121–127
Li F, Sun J, Zhu H, Wen X, Lin C, Shi D (2011) Preparation and characterization novel polymer-coated magnetic nanoparticles as carriers for doxorubicin. Colloids Surf B Biointerfaces 88:58–62
Zhang WL, Piao SH, Choi HJ (2014) Magnetic carbonyl iron suspension with sepiolite additive and its magnetorheological property. IEEE Trans Magn 50: art. No. 2000204
Fang FF, Choi HJ, Jhon MS (2009) Magnetorheology of soft magnetic carbonyl iron suspension with single-walled carbon nanotube additive and its yield stress scaling function. Colloids Surf A 351:46–51
Kim YH, Lee JE, Cho SK, Park SY, Jeong IB, Jeong MG, Kim YD, Choi HJ, Cho SM (2012) Ultrathin polydimethylsiloxane-coated carbonyl iron particles and their magnetorheological characteristics. Colloid Polym Sci 290:1093–1098
Ginder JM, Davis LC, Elie LD (1996) Rheology of magnetorheological fluids: models and measurements. Int J Mod Phys B 10:3293–3303
Sung JH, Cho MS, Choi HJ, Jhon MS (2004) Electrorheology of semiconducting polymers. J Ind Eng Chem 10:1217–1229
Liu YD, Park BJ, Fang FF, Choi HJ, Ahn WS (2013) Iron oxide/MCM-41 mesoporous nanocomposites and their magnetorheology. Colloid Polym Sci 291:1895–1901
Bombard AJF, Knobel M, Alcantara MR (2007) Phosphate coating on the surface of carbonyl iron powder and its effect in magnetorheological suspensions. Int J Mod Phys B 21:4858–4867
Acknowledgments
This work was financially supported by Ministry of Trade, Industry and Energy, Korea (no. 10047791).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Chae, H.S., Kim, S.D., Piao, S.H. et al. Core-shell structured Fe3O4@SiO2 nanoparticles fabricated by sol–gel method and their magnetorheology. Colloid Polym Sci 294, 647–655 (2016). https://doi.org/10.1007/s00396-015-3818-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-015-3818-y