Abstract
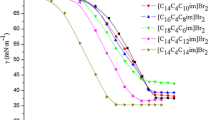
Two tetrasiloxane Gemini imidazolium surfactants with methylene spacer groups ([Si4-s-Si4im]Cl2, s = 4, 6) were synthesized and characterized by 1H NMR and ESI-MS spectrum. The surface activity and thermodynamic properties in aqueous solution among three categories of surfactants, including [Si4-s-Si4im]Cl2, the corresponding monomer ([Si4mim]Cl) and hydrocarbon-based Gemini imidazolium surfactant ([C14-4-C14im]Cl2), were compared by surface tension and electrical conductivity methods. A series of surface activity parameters, including cmc, γ cmc, π cmc, pc20, Γ max and A min, and the adsorption isotherms were obtained from the surface tension plots. The results indicated that the tetrasiloxane Gemini imidazolium surfactant with the longer spacer group has the higher capacity to form micelles but lower efficiency to reduce surface tension. Besides, the cmc value of [Si4-s-Si4im]Cl2 was about one order of magnitude lower than that of [Si4mim]Cl. The tetrasiloxane-based surfactants have the higher capacity to low the surface tension than the hydrocarbon-based surfactant. The adsorption isotherms of the tetrasiloxane-based surfactants are similar to those of conventional hydrocarbon-based surfactants. The thermodynamic parameters of micellization process, namely, the standard Gibbs free energy (ΔG m θ), enthalpy (ΔH m θ) and entropy (ΔS m θ) originated from the electrical conductivity measurements at five different temperatures, suggested that the micellization of [Si4-s-Si4im]Cl2 and [C14-4-C14im]Cl2 is entropy-driven process whereas aggregation of [Si4mim]Cl is enthalpy-driven process at the whole investigated temperatures. The dynamic light scattering results indicate that the aggregation size of [Si4-4-Si4im]Cl2 (113.6 nm) is larger than [Si4-6-Si4im]Cl2 (101.7 nm).





Similar content being viewed by others
References
Hill RM (1999) Silicone surfactants. Marcel Dekker, New York, pp 5–10
Hill RM (2002) Colloid Interface Sci 7:255–261
Hill RM (1998) Colloid Interface Sci 3:247–254
Somasundaran P, Mehta SC, Purohit P (2006) Adv Colloid Interface Sci 128:103–109
Smid-Korbar J, Kristi J, Stare M (1990) Int J Cosmet Sci 12:135–139
Svitove T, Hoffmann H, Hill RM (1996) Langmuir 12:1712–1721
Hill RM, He M, Davis HT, Scriven LE (1994) Langmuir 10:1724–1734
Peter JG (1993) Pesticide Sci 38:103–122
Zhang XD, Macosko CW, Davis HT, Nikolov AD (1999) J Colloid Interface Sci 215:270–279
Policello GA, Leatherman MD, Peng W, Rajaraman et al. ZJ (2007) U.S. Patent 20070184005
Hill RM, Svitova T, Smirnova Y, Stuermer A (1998) Langmuir 14:5023–5031
Hill RM, Churaev V, Esipova NE, Sobolev VD (2001) Langmuir 17:1338–1348
Harald W, Knudsen KD (2008) Langmuir 24:10637–10645
Natalia I, Victor S, Daniel J, Nidal H, Ramon R (2009) Langmuir 25:3564
Svitova TF, Hill RM, Radke CJ (2001) Langmuir 17:335
Li X, Washenberger RM, Scriven LE, Davis HT (1999) Langmuir 15:2278
Peng ZL, Wu Q, Cai T, Gao HY, Chen KL (2009) Colloids Surf A: Physicochem Eng Aspects 342:127–131
Tan JL, Ma DP, Feng SY, Zhang CQ (2013) Colloids Surf A: Physicochem Eng Aspects 417:146–153
Snow SA, Fenton WN, Owen MJ (1991) Langmuir 7:7868–7871
Tan JL, Zhao PJ, Ma DP, Feng SY, Zhang CQ (2013) Colloid Polym Sci 291:1487–1494
Ding YS, Zha M, Zhang J, Wang SS (2007) Chin Chem Lett 18:48
Cai YQ, Yu GQ, Liu CD (2012) Chin Chem Lett 23:1
Antonietti M, Kuang DB, Smarsly B (2004) Chem Int Ed 43:4988
Fry AJ (2003) Electroanal. J Chem 546:35
Xia HO, Yu J, Jiang YY, Mahmood I, Liu HZ (2007) Ind Eng Chem Res 46:2112
Ao MQ, Xu GY, Zhu YY, Bai YJ (2008) Colloid Interface Sci 326:490–495
Liu GY, Gu DM, Liu HY, Ding W, Li Z (2011) J Colloid Interface Sci 358:521
Ao MQ, Huang PP, Xu GY, Yang XD, Wang YJ (2009) Colloid Polym Sci 287:395–402
Ding YS, Zha M, Zhang J, Wang SS (2007) Colloids Surf A: Physicochem Eng Aspects 298:201
Cai B, Dong JF, Cheng L, Jiang Z, Yang Y, Li XF (2013) Soft Matter 9:7639
Schmaucks G, Sonnek G, Wustneck M, Ramm M (1992) Langmuir 8:1724–1730
Kamboj R, Singh S, Chauhan V (2014) Colloids Surf A: Physicochem Eng Aspects 441:233–241
Ren CC, Wang F, Zhang ZQ, Nie HH, Li N, Cui M (2015) Colloids Surf A: Physicochem Eng Aspects 467:4
Zana R (2002) Adv Colloid Interface Sci 97:205–253
Shanks CP, Franses IE (1992) J Phys Chem 96:1794–1805
Aguiar J, Molina-Bolívar JA, Peula-García JM, Ruiz CC (2002) J Colloid Interface Sci 255:382–390
Ruiz CC, Díaz-López L, Aguiar J (2007) J Colloid Interface Sci 305:293–300
Łuczak J, Jungnickel C, Joskowska M, Thöming J, Hupka J (2009) J Colloid Interface Sci 336:111–116
Inoue T, Ebina H, Dong B, Zheng LQ (2007) J Colloid Interface Sci 314:236
Bhattacharya S, Haldar J (2004) Langmuir 20:7940–7947
Tsao HK (1998) J Phys Chem B 102:10243–10247
Shimizu S, Pires PAR, El Seoud OA (2004) Langmuir 20:9551–9559
Shi LJ, Li N, Yan H, Gao YA, Zheng LQ (2011) Langmuir 27:1618
Olutas EB, Aamis MJ (2012) Chem Thermodyn 47:144–153
Zhang SH, Yan H, Zhao MW, Zheng LQ (2012) J Colloid Interface Sci 372:52–57
Acknowledgments
The authors are thankful to the teachers and classmates who offered help for the whole experimental process.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhao, X., Liang, W., An, D. et al. Synthesis and properties of tetrasiloxane Gemini imidazolium surfactants. Colloid Polym Sci 294, 491–500 (2016). https://doi.org/10.1007/s00396-015-3805-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-015-3805-3