Abstract
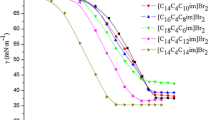
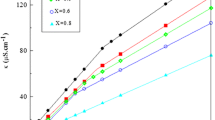
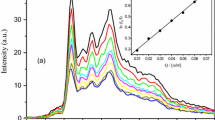
The aggregation behavior and thermodynamic properties of micellization for the ionic liquid-type gemini imidazolium surfactants with different spacer length ([C12–s–C12im]Br2, s = 2, 4, 6) have been investigated by means of surface tension, electrical conductivity, dynamic light scattering and fluorescence measurements. The values of cmc, γ cmc, Γ max, A min, π cmc, pc20 and cmc/pc20 suggest that the shorter the spacer, the higher the surface activity of [C12–s–C12im]Br2 is. The cmc and γ cmc values are decreased significantly in the presence of sodium halides, and the values decrease in the order NaCl < NaBr < NaI. The thermodynamic parameters of micellization (\(\Delta G_{\text{m}}^0 \), \(\Delta H_{\text{m}}^0 \), \(\Delta S_{\text{m}}^0 \)) indicate that the micellization of [C12–2–C12im]Br2 and [C12–4–C12im]Br2 is entropy-driven, whereas aggregation of [C12–6–C12im]Br2 is enthalpy-driven at lower temperature but entropy-driven at higher temperature. Finally, the fluorescence measurements show that the micropolarity of micelles increases but the aggregation numbers decrease with increasing the spacer length of [C12–s–C12im]Br2.







Similar content being viewed by others
References
Zana R (2002) Adv Colloid Interface Sci 97:205–253
Menger FM, Keiper JS (2000) Angew Chem Int Ed 39:1906–1920
Kim SS, Zhang WZ, Pinnavaia TJ (1998) Science 282:1302–1305
Bernd T (2005) Colloid Polym Sci 283:421–430
Bell PC, Bergsma M, Dolbnya IP et al (2003) J Am Chem Soc 125:1551–1558
De S, Aswal VK, Goyal PS, Bhattacharya S (1998) J Phys Chem B 102:6152–6160
Rosen MJ, Mathias JH, Davenport L (1999) Langmuir 15:7340–7346
Wettig SD, Verrall RE (2001) J Colloid Interface Sci 235:310–316
Wettig SD, Nowak P, Verrall RE (2002) Langmuir 18:5354–5359
Mathias JH, Rosen MJ, Davenport L (2001) Langmuir 17:6148–6154
Wang XY, Wang JB, Wang YL, Yan HK (2004) Langmuir 20:53–56
Grosmaire L, Chorro M, Chorro C et al (2002) J Colloid Interface Sci 246:175–181
Zana R (2002) J Colloid Interface Sci 248:203–220
Badea I, Wettig S, Verrall R, Foldvari M (2007) Eur J Pharm Biopharm 65:414–422
Yang H, Shen ZW, Zhou XJ (2005) Langmuir 21:10931–10940
André L, Klaus L, Rivo HR, Laurent W (2005) Colloid Polym Sci 283:469–479
Tae-Soo C, Yoshio S, Hirofusa S, Kunihiro H (2001) Dyes Pigments 50:55–65
Ao MQ, Xu GY, Zhu YY, Bai Y (2008) J Colloid Interface Sci 326:490–495
Baltazar QQ, Chandawalla J, Anderson JL (2007) Colloids Surf A 302:150–156
Ding YX, Zha M, Zhang J, Wang SS (2007) Colloids Surf A 298:201–205
Turro NJ, Yekta A (1978) J Am Chem Soc 100:5951–5952
Rosen MJ (2004) Surfactants and interfacial phenomena, 3rd edn. Wiley, New York, p p 83,149,215
Para G, Jarek E, Warszynski P (2006) Adv Colloid Interface Sci 122:39–55
Moroi Y (1992) Micelles: theoretical and applied aspects. Plenum, New York, p 61
Bhattacharya S, Haldar J (2004) Langmuir 20:7940–7947
Tsao HK (1998) J Phys Chem B 102:10243–10247
Zana R (1996) Langmuir 12:1208–1211
Nusselder JJH, Engberts JBFN (1992) J Colloid Interface Sci 148:353–361
Koyanagi M (1968) J Mol Spectrosc 25:273–290
Zana R, Levy IHM, Duportail G (1997) Langmuir 13:5552–5557
Kalyanasundaram K, Thomas JK (1997) J Am Chem Soc 99:2039–2044
Acknowledgment
The authors are grateful to the Natural Science Foundation of Shandong Province, China (Y2007B32).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ao, M., Huang, P., Xu, G. et al. Aggregation and thermodynamic properties of ionic liquid-type gemini imidazolium surfactants with different spacer length. Colloid Polym Sci 287, 395–402 (2009). https://doi.org/10.1007/s00396-008-1976-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-008-1976-x