Abstract
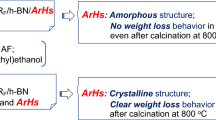
Fluoroalkyl end-capped vinyltrimethoxysilane oligomer/anatase titanium oxide nanocomposite-encapsulated low molecular weight aromatic compounds [RF-(VM-SiO2)n-RF/an-TiO2/Ar-H] were prepared by the sol–gel reactions of the corresponding oligomer in the presence of anatase titanium oxide nanoparticles (an-TiO2) and the aromatic compounds such as bisphenol A [BPA], 1,1′-bi(2-naphthol) [BINOL], and fullerene under alkaline conditions. Thermogravimetric analyses measurements show that RF-(VM-SiO2)n-RF/an-TiO2 nanocomposite-encapsulated BPA and BINOL, in which the theoretical contents in the composites are 25 ∼ 32 %, were found to give no weight loss corresponding to the contents of these aromatic compounds even after calcination at 800 °C. On the other hand, the corresponding nanocomposite-encapsulated fullerene exhibited weight loss behavior related to the presence of fullerene under similar conditions; however, UV–vis spectra showed the presence of the residual fullerene in the composites even after calcination. An-TiO2 in these fluorinated nanocomposites can keep its crystalline structure without phase transformation into rutile even after calcination at 1,000 °C, although the parent an-TiO2 nanoparticles underwent a complete phase transformation into rutile under similar conditions. Notably, RF-(VM-SiO2)n-RF/an-TiO2/Ar-H nanocomposites can give a good photocatalytic activity even after calcination at 1,000 °C for the decolorization of methylene blue under UV light irradiation. More interestingly, these fluorinated nanocomposites before and after calcination were found to exhibit a higher photocatalytic activity at the initial UV light irradiation from 1 to 3 min than that of the corresponding RF-(VM-SiO2)n-RF/an-TiO2 nanocomposites under similar conditions.

Encapsulated BPA and BINOL in the nanocomposites exhibit no weight loss even after calcination at 800 °C, and RF-(VM-SiO2)n-RF/an-TiO2/Ar-H nanocomposites before and after calcination at 1,000 °C can give a higher photocatalytic activity than that of RF-(VM-SiO2)n-RF/an-TiO2 nanocomposites. Notably, the photocatalytic activity of RF-(VM-SiO2)n-RF/an-TiO2/C60 nanocomposites after calcination increased by about 2.5-fold, compared with that of RF-(VM-SiO2)n-RF/an-TiO2 nanocomposites.
















Similar content being viewed by others
References
Arellano M, Mickel-Haciski I, Feke DL, Manas-Zloczower I (1996) J Coat Technol 68:83
Hartley PA, Parfitt GD, Pollack LB (1985) Power Technol 42:35
Millis A, Lee S-K (2000) J Photochem Photobiol A: Chem 130:163
Mills A, Hill G, Bhopal S, Parkin IP, O’Neill SA (2003) J Photochem Photobiol A: Chem 160:185
Zhu Y, Zhang L, Yao W, Cao L (2000) Appl Surf Sci 158:32
Yu JC, Yu J, Zhao J (2002) Appl Catal, B 36:31
Reddy JS, Kumar R (1991) J Catal 130:440
Sawada H, Sawada E, Kakehi H, Kariya T, Mugisawa M, Chounan Y, Miura M, Isu N (2009) Polym Compos 30:1848
Sawada E, Kakehi H, Chounan Y, Miura M, Sato Y, Isu N, Sawada H (2010) Compos Part B 41:498
Linsebigler AL, Lu GQ, Yates JT (1995) Chem Rev 95:735
Fox MA, Dulay MT (1993) Chem Rev 93:341
Karakitsou KE, Verykios XE (1993) J Phys Chem 97:1184
Hoffmann MR, Martin ST, Choi W, Bahnemann DW (1995) Chem Rev 95:69
Gratzel M (2001) Nature 414:338
O’Regan B, Gratzel M (1991) Nature 353:737
Liu S, Chen A (2005) Langmuir 21:8409
Hanaor DAH, Sorrell CC (2011) J Mater Sci 46:855
Peng X, Chen A (2006) Adv Funct Mater 16:1355
Madras G, McCoy BJ, Navrotsky A (2007) J Am Ceram Soc 90:250
Wu G, Wang J, Thoma DF, Chen A (2008) Langmuir 24:3503
Guo S, Yoshioka H, Kakehi H, Kato Y, Miura M, Isu N, Ameduri B, Sawada H (2012) J Colloid Interface Sci 387:141
Sawada H, Nakayama M (1991) J Chem Soc Chem Commun 10:677–678
Sawada H, Matsuki Y, Goto Y, Kodama S, Sugiya M, Nishiyama Y (2010) Bull Chem Soc Jpn 83:75
Sawada H, Kakehi H, Tashima T, Nishiyama Y, Miura M, Isu N (2009) J Appl Polym Sci 112:3482
Sawada H, Tashima T, Kodama S (2008) Polym Adv Technol 19:73
Sawada H, Narumi T, Kodama S, Kamijo M, Ebara R, Sugiya M, Iwasaki Y (2007) Colloid Polym Sci 285:977
Sawada H, Tashima T, Kakehi H, Nishiyama Y, Kikuchi M, Miura M, Sato Y, Isu N (2010) Polym J 42:167
Sawada H, Tashima T, Nishiyama Y, Kikuchi M, Kostov G, Goto Y, Ameduri B (2011) Macromolecules 44:1114
Sawada H, Kikuchi M, Nishida M (2011) J Polym Sci Part A; Polym Chem 49:1070
Hilonga A, Kim J-K, Sarawade PB, Kim HT (2010) J Mater Sci 45:1255
Hilonga A, Kim J-K, Sarawade PB, Kim HT (2010) J Mater Chem 45:1264
Wang Q, Chen C, Zhao D, Ma W, Zhao J (2008) Langmuir 24:7338
Liu S, Yu J, Cheng B, Jaroniec M (2012) Adv Colloid Interface Sci 173:35
Liu G, Sun C, Yang HG, Smith SC, Wang L, Lu GQ, Cheng H-M (2010) Chem Commun 46:755
Ho W, Yu JC, Lee S (2006) Chem Commun 1115
Zhang H, Liu P, Li PF, Liu H, Qang Y, Zhang S, Guo M, Cheng H, Zhao H (2011) Chem Eur J 17
Zhang D, Li G, Yang X, Yu JC (2009) Chem Commun 45:4381
Liu M, Zhao L, Ju S, Yan Z, He T, Zhou C, Wang W (2010) Chem Commun 46:1664
Han X, Kuang Q, Jin M, Xie Z, Zheng L (2009) J Am Chem Soc 131:3152
Yang GH, Liu G, Qiao SZ, Sun CH, Jin YG, Smith SC, Zou J, Cheng HM, Lu GQ (2009) J Am Chem Soc 131:4078
Li D, Haneda H, Labhsetwar NK, Hishita S, Ohashi N (2005) Chem Phys Lett 401:579
Yu JC, Yu J, Ho W, Jiang Z, Zhang L (2002) Chem Mater 14:3808
Acknowledgments
This work was partially supported by a Grant-in-Aid for Scientific Research 24550220 from the Ministry of Education, Science, Sports, and Culture, Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guo, S., Ogasawara, T., Saito, T. et al. Preparation and photocatalytic activity of fluoroalkyl end-capped vinyltrimethoxysilane oligomer/anatase titanium oxide nanocomposite-encapsulated low molecular weight aromatic compounds. Colloid Polym Sci 291, 2947–2957 (2013). https://doi.org/10.1007/s00396-013-3027-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-013-3027-5