Abstract
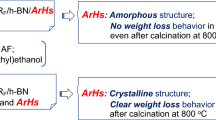
Fluoroalkyl end-capped N-(1,1-dimethyl-3-oxobutyl)acrylamide oligomer [RF-(DOBAA)n-RF]/silica gel nanocomposite, which was prepared by reaction of the corresponding fluorinated oligomer with tetraethoxysilane and silica gel nanoparticles under alkaline conditions, exhibited no weight loss even at 800 °C equal to the original silica gel, although the corresponding parent RF-(DOBAA)n-RF oligomer was completely degraded at 600 °C. Thermogravimetric analyses/mass spectra of fluorinated nanocomposite showed that this nanocomposite decomposed around 280 °C to afford CO2 and H2O as the major evolved gaseous products including some minor fluoro- and hydrocarbons. X-ray photoelectron spectroscopy analyses also showed that the contents of C, F, and Si atoms in RF-(DOBAA)n-RF/SiO2 nanocomposite after the calcination at 800 °C were similar to those before the calcination. These findings suggest that the evolved gaseous products should be encapsulated quantitatively into nanometer-size-controlled silica matrices to give the fluorinated silica gel nanocomposite with no weight loss even at 800 °C equal to the original silica gel.









Similar content being viewed by others
References
Judeinstein P, Sanchez C (1996) J Mater Chem 6:511–525
Wen J, Wilkes GL (1996) Chem Mater 8:1667–1681
Laine RM, Sanchez C, Brinker CJ, Giannelis E (1998) Organic–inorganic hybrid materials. Materials Research Society, Warrendale, PA, USA
Brinker CJ, Scherer GW (1990) Sol–gel science. Academic, Boston
Misra M, Guest A, Tilley M (1998) Surf Coat Int 81:594–595
Hua Z, Qiu K-Y (1997) Polymer 31:521–526
Pedroso MAS, Dias ML, San Gil RAS, Mothe CG (2003) Colloid Polym Sci 281:19–26
Matejka L, Plestil J (1997) Macromol Symp 122:191–196
Juangvanich N, Maurite KA (1998) J Appl Polym Sci 67:1799–1810
Johns K, Stead G (2000) J Fluorine Chem 104:5–18
Ameduri B, Boutevin B (2000) J Fluor Chem 104:53–62
Berret J-F, Calvet D, Collet A, Viguier M (2003) Curr Opin Colloid Interface Sci 8:296–306
Kujawa P, Goh CCE, Calvet D, Winnik FM (2001) Macromolecules 34:6387–6395
Imae T (2003) Curr Opin Colloid Interface Sci 8:307–314
Andruzzi L, Chiellini E, Galli G, Li X, Kang SH, Ober CK (2002) J Mater Chem 12:1684–1692
Imae T, Tabuchi H, Funayama K, Sato A, Nakamura T, Amaya N (2000) Colloid Surf A Physicochem Eng Asp 167:73–81
Jankova K, Hvilsted S (2005) J Fluorine Chem 126:241–250
Lebreton P, Ameduri B, Boutevin B, Corpart J-M (2002) Macromol Chem Phys 203:522–537
Sawada H (1996) Chem Rev 96:1779–1808
Sawada H, Kawase T (2001) Kobunshi Ronbunshu 58:147–160
Sawada H, Kawase T (2001) Kobunshi Ronbunshu 58:255–266
Sawada H (2000) J Fluorine Chem 105:219–220
Harmer MK, Farneth WE, Sun Q (1996) J Am Chem Soc 118:7708–7715
Cho J-W, Sul K-I (2001) Fiber Polym 2:135–140
Yano S, Okubo N, Takahashi K (1996) Macromol Symp 108:270–289
Fabbri P, Messori M, Montecchi M, Nannarone S, Pasquali L, Pilati F, Tonelli C, Toselli M (2006) Polymer 47:1055–1062
Sawada H, Narumi T, Kajiwara A, Ueno K, Hamazaki K (2006) Colloid Polym Sci 284:551–555
Gregg SJ, Sing KS, Sing W (1982) Adsorption, surface areas and porosity, 2nd edn. Academic, New York
Sawada H, Koizumi M, Tojo T, Ohnishi T, Tomoita T (2005) Polym Adv Technol 16:459–465
Welton T (1999) Chem Rev 99:2071–2084
Sawada H, Yoshino Y, Kurachi M, Kawase T, Takishita K, Tanedani T (2000) Polymer 41:397–4000
Acknowledgement
We thank S. Nagasawa (Bruker AXS. K. K., Japan) for TG/Mass observation and Y. Yoshida (Kratos Analytical, Japan) for XPS observation. This work was partially supported by a grant-in-aid for Scientific Research from the Ministry of Education, Science, Sports and Culture, Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sawada, H., Narumi, T., Kodama, S. et al. A fluoroalkyl end-capped N-(1,1-dimethyl-3-oxobutyl)acrylamide oligomer/silica gel nanocomposite with no weight loss even at 800 °C equal to an original silica gel. Colloid Polym Sci 285, 977–983 (2007). https://doi.org/10.1007/s00396-007-1641-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-007-1641-9