Abstract
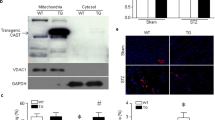
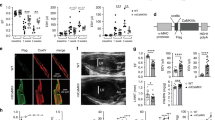
We and others have reported that calpain-1 was increased in myocardial mitochondria from various animal models of heart disease. This study investigated whether constitutive up-regulation of calpain-1 restricted to mitochondria induced myocardial injury and heart failure and, if so, whether these phenotypes could be rescued by selective inhibition of mitochondrial superoxide production. Transgenic mice with human CAPN1 up-regulation restricted to mitochondria in cardiomyocytes (Tg-mtCapn1/tTA) were generated and characterized with low and high over-expression of transgenic human CAPN1 restricted to mitochondria, respectively. Transgenic up-regulation of mitochondria-targeted CAPN1 dose-dependently induced cardiac cell death, adverse myocardial remodeling, heart failure, and early death in mice, the changes of which were associated with mitochondrial dysfunction and mitochondrial superoxide generation. Importantly, a daily injection of mitochondria-targeted superoxide dismutase mimetics mito-TEMPO for 1 month starting from age 2 months attenuated cardiac cell death, adverse myocardial remodeling and heart failure, and reduced mortality in Tg-mtCapn1/tTA mice. In contrast, administration of TEMPO did not achieve similar cardiac protection in transgenic mice. Furthermore, transgenic up-regulation of mitochondria-targeted CAPN1 induced a reduction of ATP5A1 protein and ATP synthase activity in hearts. In cultured cardiomyocytes, increased calpain-1 in mitochondria promoted mitochondrial permeability transition pore (mPTP) opening and induced cell death, which were prevented by over-expression of ATP5A1, mito-TEMPO or cyclosporin A, an inhibitor of mPTP opening. In conclusion, this study has provided direct evidence demonstrating that increased mitochondrial calpain-1 is an important mechanism contributing to myocardial injury and heart failure by disrupting ATP synthase, and promoting mitochondrial superoxide generation and mPTP opening.







Similar content being viewed by others
References
Barth E, Stammler G, Speiser B, Schaper J (1992) Ultrastructural quantitation of mitochondria and myofilaments in cardiac muscle from 10 different animal species including man. J Mol Cell Cardiol 24:669–681. https://doi.org/10.1016/0022-2828(92)93381-S
Botker HE, Hausenloy D, Andreadou I, Antonucci S, Boengler K, Davidson SM, Deshwal S, Devaux Y, Di Lisa F, Di Sante M, Efentakis P, Femmino S, Garcia-Dorado D, Giricz Z, Ibanez B, Iliodromitis E, Kaludercic N, Kleinbongard P, Neuhauser M, Ovize M, Pagliaro P, Rahbek-Schmidt M, Ruiz-Meana M, Schluter KD, Schulz R, Skyschally A, Wilder C, Yellon DM, Ferdinandy P, Heusch G (2018) Practical guidelines for rigor and reproducibility in preclinical and clinical studies on cardioprotection. Basic Res Cardiol 113:39. https://doi.org/10.1007/s00395-018-0696-8
Brown DI, Griendling KK (2015) Regulation of signal transduction by reactive oxygen species in the cardiovascular system. Circ Res 116:531–549. https://doi.org/10.1161/circresaha.116.303584
Bukowska A, Lendeckel U, Bode-Boger SM, Goette A (2012) Physiologic and pathophysiologic role of calpain: implications for the occurrence of atrial fibrillation. Cardiovasc Ther 30:e115–e127. https://doi.org/10.1111/j.1755-5922.2010.00245.x
Chen Q, Lesnefsky EJ (2015) Heart mitochondria and calpain 1: location, function, and targets. Biochim Biophys Acta 1852:2372–2378. https://doi.org/10.1016/j.bbadis.2015.08.004
Chen Q, Paillard M, Gomez L, Ross T, Hu Y, Xu A, Lesnefsky EJ (2011) Activation of mitochondrial mu-calpain increases AIF cleavage in cardiac mitochondria during ischemia-reperfusion. Biochem Biophys Res Commun 415:533–538. https://doi.org/10.1016/j.bbrc.2011.10.037
Crompton M, Ellinger H, Costi A (1988) Inhibition by cyclosporin A of a Ca2+-dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress. Biochem J 255:357–360. https://doi.org/10.2042/bj2550357
Dai DF, Chen T, Szeto H, Nieves-Cintron M, Kutyavin V, Santana LF, Rabinovitch PS (2011) Mitochondrial targeted antioxidant peptide ameliorates hypertensive cardiomyopathy. J Am Col Cardiol 58:73–82. https://doi.org/10.1016/j.jacc.2010.12.044
Dai DF, Hsieh EJ, Chen T, Menendez LG, Basisty NB, Tsai L, Beyer RP, Crispin DA, Shulman NJ, Szeto HH, Tian R, MacCoss MJ, Rabinovitch PS (2013) Global proteomics and pathway analysis of pressure-overload-induced heart failure and its attenuation by mitochondrial-targeted peptides. Circ Heart Fail 6:1067–1076. https://doi.org/10.1161/circheartfailure.113.000406
Dai DF, Johnson SC, Villarin JJ, Chin MT, Nieves-Cintron M, Chen T, Marcinek DJ, Dorn GW 2nd, Kang YJ, Prolla TA, Santana LF, Rabinovitch PS (2011) Mitochondrial oxidative stress mediates angiotensin II-induced cardiac hypertrophy and Galphaq overexpression-induced heart failure. Circ Res 108:837–846. https://doi.org/10.1161/circresaha.110.232306
Dromparis P, Michelakis ED (2013) Mitochondria in vascular health and disease. Annu Rev Physiol 75:95–126. https://doi.org/10.1146/annurev-physiol-030212-183804
Fernandez-Sanz C, Ruiz-Meana M, Castellano J, Miro-Casas E, Nunez E, Inserte J, Vazquez J, Garcia-Dorado D (2015) Altered FoF1 ATP synthase and susceptibility to mitochondrial permeability transition pore during ischaemia and reperfusion in aging cardiomyocytes. Thromb Haemost 113:441–451. https://doi.org/10.1160/TH14-10-0901
Gadicherla AK, Wang N, Bulic M, Agullo-Pascual E, Lissoni A, De Smet M, Delmar M, Bultynck G, Krysko DV, Camara A, Schluter KD, Schulz R, Kwok WM, Leybaert L (2017) Mitochondrial Cx43 hemichannels contribute to mitochondrial calcium entry and cell death in the heart. Basic Res Cardiol 112:27. https://doi.org/10.1007/s00395-017-0618-1
Galvez AS, Diwan A, Odley AM, Hahn HS, Osinska H, Melendez JG, Robbins J, Lynch RA, Marreez Y, Dorn GW 2nd (2007) Cardiomyocyte degeneration with calpain deficiency reveals a critical role in protein homeostasis. Circ Res 100:1071–1078. https://doi.org/10.1161/01.RES.0000261938.28365.11
Goll DE, Thompson VF, Li H, Wei W, Cong J (2003) The calpain system. Physiol Rev 83:731–801. https://doi.org/10.1152/physrev.00029.2002
Goncalves RL, Quinlan CL, Perevoshchikova IV, Hey-Mogensen M, Brand MD (2015) Sites of superoxide and hydrogen peroxide production by muscle mitochondria assessed ex vivo under conditions mimicking rest and exercise. J Biol Chem 290:209–227. https://doi.org/10.1074/jbc.M114.619072
Heusch G, Libby P, Gersh B, Yellon D, Bohm M, Lopaschuk G, Opie L (2014) Cardiovascular remodelling in coronary artery disease and heart failure. Lancet 383:1933–1943. https://doi.org/10.1016/S0140-6736(14)60107-0
Kang PT, Chen CL, Lin P, Chilian WM, Chen YR (2017) Impairment of pH gradient and membrane potential mediates redox dysfunction in the mitochondria of the post-ischemic heart. Basic Res Cardiol 112:36. https://doi.org/10.1007/s00395-017-0626-1
Kar P, Samanta K, Shaikh S, Chowdhury A, Chakraborti T, Chakraborti S (2010) Mitochondrial calpain system: an overview. Arch Biochem Biophys 495:1–7. https://doi.org/10.1016/j.abb.2009.12.020
Li J, Zhu H, Shen E, Wan L, Arnold JM, Peng T (2010) Deficiency of rac1 blocks NADPH oxidase activation, inhibits endoplasmic reticulum stress, and reduces myocardial remodeling in a mouse model of type 1 diabetes. Diabetes 59:2033–2042. https://doi.org/10.2337/db09-1800
Li S, Zhang L, Ni R, Cao T, Zheng D, Xiong S, Greer PA, Fan GC, Peng T (2016) Disruption of calpain reduces lipotoxicity-induced cardiac injury by preventing endoplasmic reticulum stress. Biochim Biophys Acta 1862:2023–2033. https://doi.org/10.1016/j.bbadis.2016.08.005
Li X, Li Y, Shan L, Shen E, Chen R, Peng T (2009) Over-expression of calpastatin inhibits calpain activation and attenuates myocardial dysfunction during endotoxaemia. Cardiovasc Res 83:72–79. https://doi.org/10.1093/cvr/cvp100
Li Y, Ma J, Zhu H, Singh M, Hill D, Greer PA, Arnold JM, Abel ED, Peng T (2011) Targeted inhibition of calpain reduces myocardial hypertrophy and fibrosis in mouse models of type 1 diabetes. Diabetes 60:2985–2994. https://doi.org/10.2337/db10-1333
Ma J, Wei M, Wang Q, Li J, Wang H, Liu W, Lacefield JC, Greer PA, Karmazyn M, Fan GC, Peng T (2012) Deficiency of Capn4 gene inhibits nuclear factor-kappaB (NF-kappaB) protein signaling/inflammation and reduces remodeling after myocardial infarction. J Biol Chem 287:27480–27489. https://doi.org/10.1074/jbc.M112.358929
Mann PJ, Quastel JH (1946) Toxic effects of oxygen and of hydrogen peroxide on brain metabolism. Biochem J 40:139–144. https://doi.org/10.1042/bj0400139
Merlo M, Caiffa T, Gobbo M, Adamo L, Sinagra G (2018) Reverse remodeling in dilated cardiomyopathy: insights and future perspectives. Int J Cardiol Heart Vasc 18:52–57. https://doi.org/10.1016/j.ijcha.2018.02.005
Merlo M, Cannata A, Gobbo M, Stolfo D, Elliott PM, Sinagra G (2018) Evolving concepts in dilated cardiomyopathy. Eur J Heart Fail 20:228–239. https://doi.org/10.1002/ejhf.1103
Murphy MP (2009) How mitochondria produce reactive oxygen species. Biochem J 417:1–13. https://doi.org/10.1042/BJ20081386
Ni R, Cao T, Xiong S, Ma J, Fan GC, Lacefield JC, Lu Y, Le Tissier S, Peng T (2016) Therapeutic inhibition of mitochondrial reactive oxygen species with mito-TEMPO reduces diabetic cardiomyopathy. Free Radic Biol Med 90:12–23. https://doi.org/10.1016/j.freeradbiomed.2015.11.013
Ni R, Zheng D, Wang Q, Yu Y, Chen R, Sun T, Wang W, Fan GC, Greer PA, Gardiner RB, Peng T (2015) Deletion of capn4 protects the heart against endotoxemic injury by preventing ATP synthase disruption and inhibiting mitochondrial superoxide generation. Circ Heart Fail 8:988–996. https://doi.org/10.1161/circheartfailure.115.002383
Ni R, Zheng D, Xiong S, Hill DJ, Sun T, Gardiner RB, Fan GC, Lu Y, Abel ED, Greer PA, Peng T (2016) Mitochondrial calpain-1 disrupts atp synthase and induces superoxide generation in type 1 diabetic hearts: a novel mechanism contributing to diabetic cardiomyopathy. Diabetes 65:255–268. https://doi.org/10.2337/db15-0963
Ono Y, Saido TC, Sorimachi H (2016) Calpain research for drug discovery: challenges and potential. Nat Rev Drug Discov 15:854–876. https://doi.org/10.1038/nrd.2016.212
Poncelas M, Inserte J, Aluja D, Hernando V, Vilardosa U, Garcia-Dorado D (2017) Delayed, oral pharmacological inhibition of calpains attenuates adverse post-infarction remodelling. Cardiovasc Res 113:950–961. https://doi.org/10.1093/cvr/cvx073
Sanbe A, Gulick J, Hanks MC, Liang Q, Osinska H, Robbins J (2003) Reengineering inducible cardiac-specific transgenesis with an attenuated myosin heavy chain promoter. Circ Res 92:609–616. https://doi.org/10.1161/01.RES.0000065442.64694.9F
Seidlmayer LK, Juettner VV, Kettlewell S, Pavlov EV, Blatter LA, Dedkova EN (2015) Distinct mPTP activation mechanisms in ischaemia–reperfusion: contributions of Ca2+, ROS, pH, and inorganic polyphosphate. Cardiovasc Res 106:237–248. https://doi.org/10.1093/cvr/cvv097
Sheeran FL, Pepe S (2006) Energy deficiency in the failing heart: linking increased reactive oxygen species and disruption of oxidative phosphorylation rate. Biochim Biophys Acta 1757:543–552. https://doi.org/10.1016/j.bbabio.2006.03.008
Shintani-Ishida K, Yoshida K (2015) Mitochondrial m-calpain opens the mitochondrial permeability transition pore in ischemia–reperfusion. Int J Cardiol 197:26–32. https://doi.org/10.1016/j.ijcard.2015.06.010
Sorescu D, Griendling KK (2002) Reactive oxygen species, mitochondria, and NAD(P)H oxidases in the development and progression of heart failure. Congest Heart Fail 8:132–140. https://doi.org/10.1111/j.1527-5299.2002.00717.x
Teshima Y, Takahashi N, Nishio S, Saito S, Kondo H, Fukui A, Aoki K, Yufu K, Nakagawa M, Saikawa T (2014) Production of reactive oxygen species in the diabetic heart. Roles of mitochondria and NADPH oxidase. Circ J 78:300–306. https://doi.org/10.1253/circj.CJ-13-1187
Thompson J, Hu Y, Lesnefsky EJ, Chen Q (2016) Activation of mitochondrial calpain and increased cardiac injury: beyond AIF release. Am J Physiol Heart Circ Physiol 310:H376–H384. https://doi.org/10.1152/ajpheart.00748.2015
Xu T, Ding W, Ao X, Chu X, Wan Q, Wang Y, Xiao D, Yu W, Li M, Yu F, Wang J (2019) ARC regulates programmed necrosis and myocardial ischemia/reperfusion injury through the inhibition of mPTP opening. Redox Biol 20:414–426. https://doi.org/10.1016/j.redox.2018.10.023
Yang D, Ma S, Tan Y, Li D, Tang B, Zhang X, Sun M, Yang Y (2010) Increased expression of calpain and elevated activity of calcineurin in the myocardium of patients with congestive heart failure. Int J Mol Med 26:159–164. https://doi.org/10.3892/ijmm_00000448
Zhu H, Shan L, Schiller PW, Mai A, Peng T (2010) Histone deacetylase-3 activation promotes tumor necrosis factor-alpha (TNF-alpha) expression in cardiomyocytes during lipopolysaccharide stimulation. J Biol Chem 285:9429–9436. https://doi.org/10.1074/jbc.M109.071274
Acknowledgements
This study was supported by operating grants from the National Natural Science Foundation of China (81,470,499 to T.P., 81521001 and 31570904 to R.C.), Natural Science Foundation of Jiangsu Province (BK20171216 to T.P.), and the Canadian Institutes of Health Research (MOP-133657 to T.P.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cao, T., Fan, S., Zheng, D. et al. Increased calpain-1 in mitochondria induces dilated heart failure in mice: role of mitochondrial superoxide anion. Basic Res Cardiol 114, 17 (2019). https://doi.org/10.1007/s00395-019-0726-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-019-0726-1