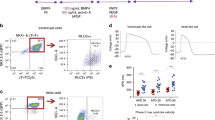
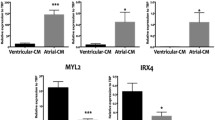
Abstract
Engineered heart tissue (EHT) from rat cells is a useful tool to study ventricular biology and cardiac drug safety. Since atrial and ventricular cells differ significantly, EHT and other 3D cell culture formats generated from ventricular cells have been of limited value to study atrial biology. To date, reliable in vitro models that reflect atrial physiology are lacking. Therefore, we established a novel EHT model using rat atrial cells (atrial EHT, aEHT) to assess atrial physiology, contractility and drug response. The tissue constructs were characterized with regard to gene expression, histology, electrophysiology, and the response to atrial-specific drugs. We observed typical functional properties of atrial tissue in our model such as more regular spontaneous beating with lower force, shorter action potential duration, and faster contraction and relaxation compared to ventricular EHT (vEHT). The expression of atrial-specific genes and proteins was high, whereas ventricle-specific transcripts were virtually absent. The atrial-selective drug carbachol had a strong negative inotropic and chronotropic effect on aEHT only. Taken together, the results demonstrate the feasibility of aEHT as a novel atrial 3D model and as a benchmark for tissue engineering with human induced pluripotent stem cell-derived atrial-like cardiomyocytes. Atrial EHT faithfully recapitulates atrial physiology and shall be useful to study atrial molecular physiology in health and disease as well as drug response.











Similar content being viewed by others
References
Amos GJ, Wettwer E, Metzger F, Li Q, Himmel HM, Ravens U (1996) Differences between outward currents of human atrial and subepicardial ventricular myocytes. J Physiol 491(1):31–50. https://doi.org/10.1113/jphysiol.1996.sp021194
Balouch M, Kolek MJ, Darbar D (2014) Improved understanding of the pathophysiology of atrial fibrillation through the lens of discrete pathological pathways. Glob Cardiol Sci Pract 5:24–36. https://doi.org/10.5339/gcsp.2014.5
Barth AS, Merk S, Arnoldi E, Zwermann L, Kloos P, Gebauer M, Steinmeyer K, Bleich M, Kääb S, Pfeufer A, Überfuhr P, Dugas M, Steinbeck G, Nabauer M (2005) Functional profiling of human atrial and ventricular gene expression. Pflugers Arch Eur J Physiol 450:201–208. https://doi.org/10.1007/s00424-005-1404-8
Burashnikov A, Antzelevitch C (2006) Late-phase 3 EAD. A unique mechanism contributing to initiation of atrial fibrillation. Pacing Clin Electrophysiol 29:290–295. https://doi.org/10.1111/j.1540-8159.2006.00336.x
Christ T, Galindo-Tovar A, Thoms M, Ravens U, Kaumann AJ (2009) Inotropy and L-type Ca 2 + current, activated by β1- and β2-adrenoceptors, are differently controlled by phosphodiesterases 3 and 4 in rat heart. Br J Pharmacol 156:62–83. https://doi.org/10.1111/j.1476-5381.2008.00015.x
Cyganek L, Tiburcy M, Sekeres K, Gerstenberg K, Bohnenberger H, Lenz C, Henze S, Salinas MSG, Zimmermann W-H, Hasenfuss G, Guan K (2018) Deep phenotyping of human induced pluripotent stem cell—derived atrial and ventricular cardiomyocytes. J Clin Investig. https://doi.org/10.1172/jci.insight.99941DS1
Devalla HD, Schwach V, Ford JW, Milnes JT, El-Haou S, Jackson C, Gkatzis K, Elliott DA, de Sousa Chuva, Lopes SM, Mummery CL, Verkerk AO, Passier R (2015) Atrial-like cardiomyocytes from human pluripotent stem cells are a robust preclinical model for assessing atrial-selective pharmacology. EMBO Mol Med 7:394–410. https://doi.org/10.15252/emmm.201404757
Eder A, Vollert I, Hansen A, Eschenhagen T (2016) Human engineered heart tissue as a model system for drug testing. Adv Drug Deliv Rev 96:214–224. https://doi.org/10.1016/j.addr.2015.05.010
Ghiroldi A, Piccoli M, Ciconte G, Pappone C, Anastasia L (2017) Regenerating the human heart : direct reprogramming strategies and their current limitations. Basic Res Cardiol 112:1–14. https://doi.org/10.1007/s00395-017-0655-9
Godier-Furnémont AFG, Tiburcy M, Wagner E, Dewenter M, Lämmle S, El-Armouche A, Lehnart SE, Vunjak-Novakovic G, Zimmermann W-H (2015) Physiologic force-frequency in engineered heart muscle by electromechanical stimulation. Biomaterials 60:82–91. https://doi.org/10.1016/j.biomaterials.2015.03.055
Grandi E, Pandit SV, Voigt N, Workman AJ, Dobrev D, Jalife J, Bers DM (2011) Human atrial action potential and Ca2+ model: sinus rhythm and chronic atrial fibrillation. Circ Res 109:1055–1066. https://doi.org/10.1161/CIRCRESAHA.111.253955
Grimm M, El-Armouche A, Zhang R, Anderson ME, Eschenhagen T (2007) Reduced contractile response to alpha1-adrenergic stimulation in atria from mice with chronic cardiac calmodulin kinase II inhibition. J Mol Cell Cardiol 42:643–652. https://doi.org/10.1016/j.yjmcc.2006.12.010
Hansen A, Eder A, Bönstrup M, Flato M, Mewe M, Schaaf S, Aksehirlioglu B, Schwörer A, Uebeler J, Eschenhagen T (2010) Development of a drug screening platform based on engineered heart tissue. Circ Res 107:35–44. https://doi.org/10.1161/CIRCRESAHA.109.211458
Harvey RD, Belevych AE (2003) Muscarinic regulation of cardiac ion channels. Br J Pharmacol 139:1074–1084. https://doi.org/10.1038/sj.bjp.0705338
Heijman J, Dobrev D (2014) Rat engineered heart tissue: a novel tool in the safety pharmacology toolkit? Basic Res Cardiol 109:437. https://doi.org/10.1007/s00395-014-0437-6
Hirt MN, Boeddinghaus J, Mitchell A, Schaaf S, Boernchen C, Mueller C, Schulz H, Hubner N, Stenzig J, Stoehr A, Neuber C, Eder A, Luther PK, Hansen A, Eschenhagen T (2014) Functional improvement and maturation of rat and human engineered heart tissue by chronic electrical stimulation. J Mol Cell Cardiol 74:151–161. https://doi.org/10.1016/j.yjmcc.2014.05.009
Jackman CP, Carlson AL, Bursac N (2016) Dynamic culture yields engineered myocardium with near-adult functional output. Biomaterials 111:66–79. https://doi.org/10.1016/j.biomaterials.2016.09.024
Jungen C, Scherschel K, Eickholt C, Kuklik P, Klatt N, Bork N, Salzbrunn T, Alken F, Angendohr S, Klene C, Mester J, Klöcker N, Veldkamp MW, Schumacher U, Willems S, Nikolaev VO, Meyer C (2017) Disruption of cardiac cholinergic neurons enhances susceptibility to ventricular arrhythmias. Nat Commun 27:14155. https://doi.org/10.1038/ncomms14155
Kurian T, Ambrosi C, Hucker W, Fedorov VV, Efimov IR (2010) Anatomy and electrophysiology of the human AV node. PACE Pacing Clin Electrophysiol 33:754–762. https://doi.org/10.1111/j.1540-8159.2010.02699.x
Lee JH, Protze SI, Laksman Z, Backx PH, Keller GM (2017) Human pluripotent stem cell-derived atrial and ventricular cardiomyocytes develop from distinct mesoderm populations. Cell Stem Cell 21(179–194):e4. https://doi.org/10.1016/j.stem.2017.07.003
Lemoine MD, Krause T, Koivumäki JT, Prondzynski M, Schulze ML, Girdauskas E, Willems S, Hansen A, Christ TET (2018) Human iPSC-derived engineered heart tissue as a sensitive test system for QT prolongation and arrhythmic triggers. Circ Arrhythmia Electrophysiol. https://doi.org/10.1161/CIRCEP.117.006035
Lemoine MD, Mannhardt I, Breckwoldt K, Prondzynski M, Flenner F, Ulmer B, Hirt MN, Neuber C, Horváth A, Kloth B, Reichenspurner H, Willems S, Hansen A, Eschenhagen T, Christ T (2017) Human iPSC-derived cardiomyocytes cultured in 3D engineered heart tissue show physiological upstroke velocity and sodium current density. Sci Rep 7:5464. https://doi.org/10.1038/s41598-017-05600-w
Magnani JW, Rienstra M, Lin H, Sinner MF, Lubitz S, Mcmanus DD, Dupuis J, Ellinor PT, Benjamin EJ (2011) Atrial fibrillation: current knowledge and future directions in epidemiology and genomics. Circulation 124:1982–1993. https://doi.org/10.1161/CIRCULATIONAHA.111.039677
Malan D, Zhang M, Stallmeyer B, Mu J, Fleischmann BK, Philipp ES, Boris S (2016) Human iPS cell model of type 3 long QT syndrome recapitulates drug-based phenotype correction. Basic Res Cardiol. https://doi.org/10.1007/s00395-016-0530-0
Mesirca P, Alig J, Torrente AG, Müller JC, Marger L, Rollin A, Marquilly C, Vincent A, Dubel S, Bidaud I, Fernandez A, Seniuk A, Engeland B, Singh J, Miquerol L, Ehmke H, Eschenhagen T, Nargeot J, Wickman K, Isbrandt D, Mangoni ME (2015) Cardiac arrhythmia induced by genetic silencing of “funny” (f) channels is rescued by GIRK4 inactivation. Nat Commun 5:4664. https://doi.org/10.1038/ncomms5664
Neumann T, Ravens U, Heusch G (1998) Characterization of excitation–contraction coupling in conscious dogs with pacing-induced heart failure. Cardiovasc Res 37(2):456–466
Ng SYSY, Wong CKCK, Tsang SYSY (2010) Differential gene expressions in atrial and ventricular myocytes: insights into the road of applying embryonic stem cell-derived cardiomyocytes for future therapies. Am J Physiol Physiol 299:C1234–C1249. https://doi.org/10.1152/ajpcell.00402.2009
Page RL, Tilsch TW, Connolly SJ, Schnell DJ, Marcello SR, Wilkinson WE, Pritchett ELC (2003) Asymptomatic or “silent” atrial fibrillation frequency in untreated patients and patients receiving azimilide. Circulation 107:1141–1145. https://doi.org/10.1161/01.CIR.0000051455.44919.73
Qi XY, Yeh YH, Chartier D, Xiao L, Tsuji Y, Brundel BJJM, Kodama I, Nattel S (2009) The calcium/calmodulin/kinase system and arrhythmogenic after depolarizations in bradycardia-related acquired long-QT syndrome. Circ Arrhythmia Electrophysiol 2:295–304. https://doi.org/10.1161/CIRCEP.108.815654
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675. https://doi.org/10.1038/nmeth.2089
Schotten U, Verheule S, Kirchhof P, Goette A (2011) Pathophysiological mechanisms of atrial fibrillation: a translational appraisal. Physiol Rev 91:265–325. https://doi.org/10.1152/physrev.00031.2009
Smyrnias I, Mair W, Harzheim D, Walker SA, Roderick HL, Bootman MD (2010) Cell Calcium Comparison of the T-tubule system in adult rat ventricular and atrial myocytes, and its role in excitation–contraction coupling and inotropic stimulation. Cell Calcium 47:210–223. https://doi.org/10.1016/j.ceca.2009.10.001
Staerk L, Sherer JA, Ko D, Benjamin EJ, Helm RH (2017) Atrial fibrillation. Circ Res 120:1501–1517. https://doi.org/10.1161/CIRCRESAHA.117.309732
Stoehr A, Neuber C, Baldauf C, Vollert I, Friedrich FW, Flenner F, Carrier L, Eder A, Schaaf S, Hirt MN, Aksehirlioglu B, Tong CW, Moretti A, Eschenhagen T, Hansen A (2014) Automated analysis of contractile force and Ca2+ transients in engineered heart tissue. AJP Heart Circ Physiol 306:H1353–H1363. https://doi.org/10.1152/ajpheart.00705.2013
Szigligeti P, Pankucsi C, Bányász T, Varró A, Nánási PP (1996) Action potential duration and force-frequency relationship in isolated rabbit, guinea pig and rat cardiac muscle. J Comp Physiol B 166:150–155
Trayanova NA, Boyle PM, Arevalo HJ, Zahid S (2014) Exploring susceptibility to atrial and ventricular arrhythmias resulting from remodeling of the passive electrical properties in the heart: a simulation approach. Front Physiol 5:1–12. https://doi.org/10.3389/fphys.2014.00435
Valverde CA, Mundiña-Weilenmann C, Said M, Ferrero P, Vittone L, Salas M, Palomeque J, Petroff MV, Mattiazzi A (2005) Frequency-dependent acceleration of relaxation in mammalian heart: a property not relying on phospholamban and SERCA2a phosphorylation. J Physiol 562:801–813. https://doi.org/10.1113/jphysiol.2004.075432
Vandecasteele G, Eschenhagen T, Scholz H, Stein B, Verde I, Fischmeister R (1999) Muscarinic and beta-adrenergic regulation of heart rate, force of contraction and calcium current is preserved in mice lacking endothelial nitric oxide synthase. Nat Med 5:331–334. https://doi.org/10.1038/6553
Weinberger F, Mannhardt I, Eschenhagen T (2017) Engineering cardiac muscle tissue. Circ Res 120:1487–1500. https://doi.org/10.1161/CIRCRESAHA.117.310738
Xie Y, Sato D, Garfinkel A, Qu Z, Weiss JN (2010) So little source, so much sink: requirements for after depolarizations to propagate in tissue. Biophys J 99:1408–1415. https://doi.org/10.1016/j.bpj.2010.06.042
Yeh YH, Lemola K, Nattel S (2007) Vagal atrial fibrillation. Acta Cardiol Sin 23:1–12. https://doi.org/10.1161/CIRCULATIONAHA.107.737023
Acknowledgements
The authors want to thank Klaus Söhren, June Uebeler, Thomas Schulze, Tim Hartmann, and Grit Höppner for expert technical assistance. We additionally thank Tobias Krause for help with electrophysiological characterization, Alexander Fischer for imaging, Kristin Hartmann at Mouse Pathology Core Facility in Hamburg for histological staining, the team at FACS-Sorting Core Facility in Hamburg for their service and help regarding flow cytometry, and the UKE Microscopy Imaging Facility.
Funding
This work was supported by funding from the DZHK (German Centre for Cardiovascular Research), the German Ministry of Education and Research (BMBF) and the e:Med symAtrial consortium, a BMBF initiative.
Author information
Authors and Affiliations
Contributions
All authors participated in the design of the experiments. JK performed atrial cell isolation and EHT preparation. AL performed force frequency experiments and histology. JK and AL performed drug response experiments, gene expression analysis, and flow cytometry. Contractility was assessed by JK, AL and JS. JK and KS performed whole-mount immunostainings. All electrophysiological experiments were done by MDL, with help from Tobias Krause and TC. Rat contractility data was contributed by Klaus Söhren and TC. JK, AL, JS and TE wrote the manuscript.
Corresponding author
Ethics declarations
Ethical standards
All animal work was conducted in accordance with the Guide for the Care and Use of Laboratory Animals as adopted by the United States National Institutes of Health (NIH publication No. 85-23, revised 1996). All animal work was approved by the local Animal Welfare Committee of the City of Hamburg, Germany (approval #08/14). The manuscript does not contain clinical studies or patient data.
Conflict of interest
TE is cofounder of EHT Technologies GmbH Hamburg, which provides technical equipment for making and video-optical analysis of EHTs.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1 Optimization of protocols.
Flow cytometric analysis of cell quality after both atrial and ventricular cell preparation. a) Cell viability was analyzed by staining with the fixable viability dye eFluor450. b) Cardiomyocyte content was determined by staining for cardiac troponin T. c) Effect of mitosis inhibition on EHT culture. Comparison of one-time only and weekly treatment with AraC with regard to d) cultivation duration and e) stability of resting length. f) Relative transcript levels of fibroblast marker genes after culture period for verification of AraC effect on fibroblast proliferation. Transcript levels were normalized to Gusb transcript levels. Mean ± SEM, unpaired t-test, ***p<0.001, numbers in brackets refer to total number of EHTs/number of batches. Fig. S2 EHT morphology. a) and b) representative H&E staining of vEHT and aEHT paraffin sections. c) Alpha-actin stained aEHT paraffin section, shown in higher magnification in d). Fig. S3 Expression analysis of cell-type markers. Overview of all analyzed atrial-specific a) – d), ventricular-specific e) – j), and pacemaker genes k) - l). For Pitx2 (c) transcript levels were measured in left and right atria separately (LA and RA, respectively) to show chamber-specific expression. Error bars show mean ± SEM, EHT numbers refer to total number of EHTs/number of batches. Fig. S4 Batch-to-batch variability. Comparison of five vEHT and seven aEHT batches at day 14-15 with regard to a) frequency, b) force generation, c) contraction time, and d) relaxation time. Error bars show mean ± SEM, no significant differences between the batches. Fig. S5 Protocol overview. Graphical illustration of the aEHT preparation protocol including approximate time needed for each step. Tab. S1 Primer sequences. Overview of all genes analyzed with qPCR with according primer sequences. Tab. S2 Cell type-specific markers. Markers were chosen for gene expression analysis in the atrial and ventricular EHT model. (DOCX 2080 kb)
Rights and permissions
About this article
Cite this article
Krause, J., Löser, A., Lemoine, M.D. et al. Rat atrial engineered heart tissue: a new in vitro model to study atrial biology. Basic Res Cardiol 113, 41 (2018). https://doi.org/10.1007/s00395-018-0701-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-018-0701-2