Abstract
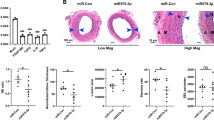
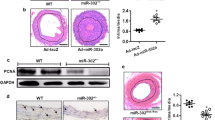
The role of miR-92a on vascular remodelling after injury is currently unknown. Thus, the aim of the present study was to evaluate the role of miR-92a on rat endothelial and vascular smooth muscle cells proliferation and migration in vitro as well as after balloon injury or arterial stenting in vivo. MiR-92a was highly expressed in RAO-ECs and vascular endothelium, but not in RAO-SMCs or medial smooth muscle as assessed by real-time RT-PCR. Importantly, BrdU incorporation and wound healing assay provide evidence that functional inhibition of miR-92a resulted in an increased RAO-ECs proliferation and migration, but had no effect on RAO-SMCs proliferation or migration in vitro. Immunoblotting analysis revealed an increased phosphorylation of ERK1/2, JNK/SAPK as well as eNOS and phospho-eNOS increased expression level in RAO-ECs as a consequence of miR-92a inhibition. Using gain and loss of function experiments, we showed that miR-92a modulates regulation of KLF4 and MKK4 expression level in endothelial cells. Finally, in vivo administration of antagomiR-92a significantly enhanced re-endothelialization in injured carotid arteries and reduced neointimal formation after balloon injury or arterial stenting. These data provide the first evidence that inhibition of miR-92a may represent a novel strategy to improve endothelial regeneration and reduce restenosis after vascular injury.






Similar content being viewed by others
References
Ambros V (2004) The functions of animal microRNAs. Nature 431:350–355. doi:10.1038/nature02871
Baker M, Robinson SD, Lechertier T, Barber PR, Tavora B, D’Amico G, Jones DT, Vojnovic B, Hodivala-Dilke K (2011) Use of the mouse aortic ring assay to study angiogenesis. Nat Protoc 22(7):89–104. doi:10.1038/nprot.2011.435
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297. doi:10.1016/S0092-8674(04)00045-5
Bavry AA, Kumbhani DJ, Helton TJ, Bhatt DL (2005) Risk of thrombosis with the use of sirolimus-eluting stents for percutaneous coronary intervention (from registry and clinical trial data). Am J Cardiol 95:1469–1472. doi:10.1016/j.amjcard.2005.02.015
Bonauer A, Carmona G, Iwasaki M, Mione M, Koyanagi M, Fischer A, Burchfield J, Fox H, Doebele C, Ohtani K, Chavakis E, Potente M, Tjwa M, Urbich C, Zeiher AM, Dimmeler S (2009) MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science 324:1710–1713. doi:10.1126/science.1174381
Camenzind E, Steg PG, Wijns W (2007) Stent thrombosis late after implantation of first-generation drug-eluting stents: a cause for concern. Circulation 115:1440–1455. doi:10.1161/CIRCULATIONAHA.106.666800
Carmeliet P, Jain RK (2011) Molecular mechanisms and clinical applications of angiogenesis. Nature 19(473):298–307. doi:10.1038/nature10144
Chai J, Jones MK, Tarnawski AS (2004) Serum response factor is a critical requirement for VEGF signaling in endothelial cells and VEGF-induced angiogenesis. FASEB J 18:1264–1266. doi:10.1096/fj.03-1232fje
Cowan CE, Kohler EE, Dugan TA, Mirza MK, Malik AB, Wary KK (2010) Kruppel-like factor-4 transcriptionally regulates VE-cadherin expression and endothelial barrier function. Circ Res 107:959–966. doi:10.1161/CIRCRESAHA.110.219592
Dews M, Homayouni A, Yu D, Murphy D, Sevignani C, Wentzel E, Furth EE, Lee WM, Enders GH, Mendell JT, Thomas-Tikhonenko A (2006) Augmentation of tumor angiogenesis by a Myc-activated microRNA cluster. Nat Genet 38:1060–1065. doi:10.1038/ng1855
Drexler H (1999) Nitric oxide and coronary endothelial dysfunction in humans. Cardiovasc Res 43:572–579. doi:10.1016/S0008-6363(99)00152-2
Fleming Y, Armstrong CG, Morrice N, Paterson A, Goedert M, Cohen P (2000) Synergistic activation of stress-activated protein kinase 1/c-Jun N-terminal kinase (SAPK1/JNK) isoforms by mitogen-activated protein kinase kinase 4 (MKK4) and MKK7. Biochem J 352:145–154
Forough R, Koyama N, Hasenstab D, Lea H, Clowes M, Nikkari ST, Clowes AW (1996) Overexpression of tissue inhibitor of matrix metalloproteinase-1 inhibits vascular smooth muscle cell functions in vitro and in vivo. Circ Res 79:812–820. doi:10.1161/01.RES.79.4.812
Friedrich EB, Werner C, Walenta K, Böhm M, Scheller B (2009) Role of extracellular signal-regulated kinase for endothelial progenitor cell dysfunction in coronary artery disease. Basic Res Cardiol 104:613–620. doi:10.1007/s00395-009-0022-6
Hamik A, Lin Z, Kumar A, Balcells M, Sinha S, Katz J, Feinberg MW, Gerzsten RE, Edelman ER, Jain MK (2007) Kruppel-like factor 4 regulates endothelial inflammation. J Biol Chem 282:13769–13779. doi:10.1074/jbc.M700078200
He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S, Powers S, Cordon-Cardo C, Lowe SW, Hannon GJ, Hammond SM (2005) A microRNA polycistron as a potential human oncogene. Nature 435:828–833. doi:10.1038/nature03552
Hoffmann R, Mintz GS, Mehran R, Pichard AD, Kent KM, Satler LF, Popma JJ, Wu H, Leon MB (1998) Intravascular ultrasound predictors of angiographic restenosis in lesions treated with Palmaz-Schatz stents. J Am Coll Cardiol 31:43–49. doi:10.1016/S0002-9149(99)00769-9
Indolfi C, Avvedimento VE, Di Lorenzo E, Esposito G, Rapacciuolo A, Giuliano P, Grieco D, Cavuto L, Stingone AM, Ciullo I, Condorelli G, Chiariello M (1997) Activation of cAMP-PKA signaling in vivo inhibits smooth muscle cell proliferation induced by vascular injury. Nat Med 3:775–779. doi:10.1038/nm0797-775
Indolfi C, Avvedimento EV, Rapacciuolo A, Di Lorenzo E, Esposito G, Stabile E, Feliciello A, Mele E, Giuliano P, Condorelli G (1995) Inhibition of cellular Ras prevents smooth muscle cell proliferation after vascular injury in vivo. Nat Med 1:541–545. doi:10.1038/nm0695-541
Indolfi C, Avvedimento EV, Rapacciuolo A, Esposito G, Di Lorenzo E, Leccia A, Pisani A, Chieffo A, Coppola A, Chiariello M (1997) In vivo gene transfer: prevention of neointima formation by inhibition of mitogen-activated protein kinase kinase. Basic Res Cardiol 92:378–384. doi:10.1007/BF00796211
Indolfi C, Di Lorenzo E, Rapacciuolo A, Stingone AM, Stabile E, Leccia A, Torella D, Caputo R, Ciardiello F, Tortora G, Chiariello M (2000) 8-chloro-cAMP inhibits smooth muscle cell proliferation in vitro and neointima formation induced by balloon injury in vivo. J Am Coll Cardiol 36:288–293. doi:10.1016/S0735-1097(00)00679-3
Indolfi C, Esposito G, Di Lorenzo E, Rapacciuolo A, Feliciello A, Porcellini A, Avvedimento VE, Condorelli M, Chiariello M (1995) Smooth muscle cell proliferation is proportional to the degree of balloon injury in a rat model of angioplasty. Circulation 92:1230–1235. doi:10.1161/01.CIR.92.5.1230
Indolfi C, Esposito G, Stabile E, Cavuto L, Pisani A, Coppola C, Torella D, Perrino C, Di Lorenzo E, Curcio A, Palombini L, Chiariello M (2000) A new rat model of small vessel stenting. Basic Res Cardiol 95:179–185. doi:10.1007/s003950050180
Indolfi C, Gasparri C, Vicinanza C, De Serio D, Boncompagni D, Mongiardo A, Spaccarotella C, Agosti V, Torella D, Curcio A (2011) Mitogen-activated protein kinases activation in T lymphocytes of patients with acute coronary syndromes. Basic Res Cardiol 106:667–679. doi:10.1007/s00395-011-0172-1
Indolfi C, Pavia M, Angelillo IF (2005) Drug-eluting stents versus bare metal stents in percutaneous coronary interventions (a meta-analysis). Am J Cardiol 95:1146–1152. doi:10.1016/j.amjcard.2005.01.040
Indolfi C, Torella D, Coppola C, Curcio A, Rodriguez F, Bilancio A, Leccia A, Arcucci O, Falco M, Leosco D, Chiariello M (2002) Physical training increases eNOS vascular expression and activity and reduces restenosis after balloon angioplasty or arterial stenting in rats. Circ Res 91:1190–1197. doi:10.1161/01.RES.0000046233.94299.D6
Kleinbongard P, Heusch G, Schulz R (2010) TNFalpha in atherosclerosis, myocardial ischemia/reperfusion and heart failure. Pharmacol Ther 127:295–314. http://dx.doi.org/10.1016/j.pharmthera.2010.05.002
Liang CC, Park AY, Guan JL (2007) In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc 2:329–333. doi:10.1038/nprot.2007.30
Liu MW, Roubin GS, King SB (1989) Restenosis after coronary angioplasty. Potential biologic determinants and role of intimal hyperplasia. Circulation 79:1374–1387. doi:10.1161/01.CIR.79.6.1374
Lorenzen JM, Martino F, Thum T (2012) Epigenetic modifications in cardiovascular disease. Basic Res Cardiol 107:245. doi:10.1007/s00395-012-0245-9
Marx SO, Totary-Jain H, Marks AR (2011) Vascular smooth muscle cell proliferation in restenosis. Circ Cardiovasc Interv 4:104–111. doi:10.1161/CIRCINTERVENTIONS.110.957332
Meder B, Keller A, Vogel B, Haas J, Sedaghat-Hamedani F, Kayvanpour E, Just S, Borries A, Rudloff J, Leidinger P, Meese E, Katus HA, Rottbauer W (2011) MicroRNA signatures in total peripheral blood as novel biomarkers for acute myocardial infarction. Basic Res Cardiol 106:13–23. doi:10.1007/s00395-010-0123-2
Nam D, Ni CW, Rezvan A, Suo J, Budzyn K, Llanos A, Harrison D, Giddens D, Jo H (2009) Partial carotid ligation is a model of acutely induced disturbed flow, leading to rapid endothelial dysfunction and atherosclerosis. Am J Physiol Heart Circ Physiol 297:1535–1543. doi:10.1152/ajpheart.00510.2009
Oppermann M, Suvorava T, Freudenberger T, Dao VT, Fischer JW, Weber M, Kojda G (2011) Regulation of vascular guanylyl cyclase by endothelial nitric oxide-dependent posttranslational modification. Basic Res Cardiol 106:539–549. doi:10.1007/s00395-011-0160-5
Ohtani K, Dimmeler S (2011) Control of cardiovascular differentiation by microRNAs. Basic Res Cardiol 106:5–11. doi:10.1007/s00395-010-0139-7
Qi YX, Jiang J, Jiang XH, Wang XD, Ji SY, Han Y, Long DK, Shen BR, Yan ZQ, Chien S, Jiang ZL (2011) PDGF-BB and TGF-{beta}1 on cross-talk between endothelial and smooth muscle cells in vascular remodeling induced by low shear stress. Proc Natl Acad Sci USA 108:1908–1913. doi:10.1073/pnas.1019219108
Rajewsky N (2006) MicroRNA target predictions in animals. Nat Genet 38:S8–S13. doi:10.1038/ng1798
Ross R (1993) The pathogenesis of atherosclerosis: a perspective for the 1990 s. Nature 362:801–809. doi:10.1038/362801a0
Salameh A, Galvagni F, Bardelli M, Bussolino F, Oliviero S (2005) Direct recruitment of CRK and GRB2 to VEGFR-3 induces proliferation, migration, and survival of Endothelial cells through the activation of ERK, AKT, and JNK pathways. Blood 106:3423–3431. doi:10.1182/blood-2005-04-1388
Sivritas D, Becher MU, Ebrahimian T, Arfa O, Rapp S, Bohner A, Mueller CF, Umemura T, Wassmann S, Nickenig G, Wassmann K (2011) Antiproliferative effect of estrogen in vascular smooth muscle cells is mediated by Kruppel-like factor-4 and manganese superoxide dismutase. Basic Res Cardiol 106:563–575. doi:10.1007/s00395-011-0174-z
Small EM, Olson EN (2011) Pervasive roles of microRNAs in cardiovascular biology. Nature 469:336–342. doi:10.1038/nature09783
Suehiro J, Hamakubo T, Kodama T, Aird WC, Minami T (2010) Vascular endothelial growth factor activation of endothelial cells is mediated by early growth response-3. Blood 115:2520–2532. doi:10.1182/blood-2009-07-233478
Torella D, Iaconetti C, Catalucci D, Ellison GM, Leone A, Waring CD, Bochicchio A, Vicinanza C, Aquila I, Curcio A, Condorelli G, Indolfi C (2011) MicroRNA-133 controls vascular smooth muscle cell phenotypic switch in vitro and vascular remodeling in vivo. Circ Res 109:880–893. doi:10.1161/CIRCRESAHA.111.240150
Walter DH, Cejna M, Diaz-Sandoval L, Willis S, Kirkwood L, Stratford PW, Tietz AB, Kirchmair R, Silver M, Curry C, Wecker A, Yoon YS, Heidenreich R, Hanley A, Kearney M, Tio FO, Kuenzler P, Isner JM, Losordo DW (2004) Local gene transfer of phVEGF-2 plasmid by gene-eluting stents: an alternative strategy for inhibition of restenosis. Circulation 110:36–45. doi:10.1161/01.CIR.0000133324.38115.0A
Wu W, Xiao H, Laguna-Fernandez A, Villarreal G Jr, Wang KC, Geary GG, Zhang Y, Wang WC, Huang HD, Zhou J, Li YS, Chien S, Garcia-Cardena G, Shyy JY (2011) Flow-dependent regulation of Kruppel-like factor 2 is mediated by MicroRNA-92a. Circulation 124:633–641. doi:10.1161/CIRCULATIONAHA.110.005108
Zhang W, Liu HT (2002) MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res 12:9–18. doi:10.1038/sj.cr.7290105
Zhang C, Wu J, Xu X, Potter BJ, Gao X (2010) Direct relationship between levels of TNF-alpha expression and endothelial dysfunction in reperfusion injury. Basic Res Cardiol 105:453–464. doi:10.1007/s00395-010-0083-6
Acknowledgments
This manuscript was dedicated to the memory of Massimo Chiariello, MD. This work was supported by the Italian Ministry of Health (Ricerca Finalizzata 2007, APICE Project) and Cardiovascular Research Association (Genecor).
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Additional information
C. Iaconetti and A. Polimeni have contributed equally to this manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Iaconetti, C., Polimeni, A., Sorrentino, S. et al. Inhibition of miR-92a increases endothelial proliferation and migration in vitro as well as reduces neointimal proliferation in vivo after vascular injury. Basic Res Cardiol 107, 296 (2012). https://doi.org/10.1007/s00395-012-0296-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-012-0296-y