Abstract
NADPH oxidase family enzymes (or NOXs) are the major sources of reactive oxygen species (ROS) that are implicated in the pathophysiology of many cardiovascular diseases. These enzymes appear to be especially important in the modulation of redox-sensitive signalling pathways that underlie key cellular functions such as growth, differentiation, migration and proliferation. Seven distinct members of the family have been identified of which four (namely NOX1, 2, 4 and 5) may have cardiovascular functions. In this article, we review our current understanding of the roles of NOX enzymes in several common cardiovascular disease states, with a focus on data from genetic studies and clinical data where available.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reactive oxygen species (ROS) have long been known to be capable of inducing detrimental effects through the oxidation and damage of cellular macromolecules, membranes, proteins and DNA, when they overwhelm endogenous antioxidant defences—a condition known as oxidative stress. More recently, however, it has been appreciated that they also modulate specific cellular signalling pathways through targeted effects on susceptible molecules, especially when generated in lower amounts—an action that is termed “redox signalling” [25, 56]. Redox signalling and oxidative stress are important in numerous cardiovascular conditions such as atherosclerosis, hypertension and heart failure [28, 30, 72, 85].
Potential sources of cellular ROS include mitochondria, NADPH oxidase family enzymes (or NOXs), dysfunctional nitric oxide (NO) synthases, xanthine oxidase and other oxygenases. Among these sources, the NOXs are unique in that ROS production is their primary function rather than a secondary effect [7]. Furthermore, they appear to be especially important in the modulation of redox-sensitive signalling pathways that underlie key cellular functions such as growth, differentiation, migration and proliferation. A large body of work published over the last decade indicates that NOX enzymes play important roles in the pathophysiology of many cardiovascular diseases [12, 16, 69]. Over this period, much has been learnt about the complexity of these enzymes, their regulation and their involvement in specific pathological processes. The most compelling data derive from studies that have used gene-modified animal models or specific genetic approaches to define the functions of these enzymes since chemical inhibitors of the enzymes lack specificity [2, 47]. Here, we provide a brief review of the current understanding of the roles of NOX enzymes in several common cardiovascular disease states, with a focus on data from genetic studies. We also review clinical data supporting a role for NOXs, where available.
NADPH oxidase structure and regulation
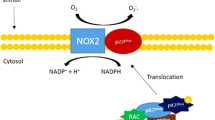
The NOX family oxidases comprise seven members, each based on a distinct core catalytic subunit; i.e. NOX1–5 and DUOX1–2 [7, 66]. All NOX enzymes utilize NADPH as an electron donor and catalyse transfer of electrons to molecular oxygen to generate superoxide (O2 ·−) and/or hydrogen peroxide (H2O2). NOX 1, 2, 4 and 5 may be expressed in cardiovascular cells although NOX5 is only found in humans and not rodents. The enzymes have differing requirements for other subunits and also exhibit differences in activity (Fig. 1; Table 1). NOX1, 2 and 4 all bind to a smaller p22phox subunit, which is essential for enzyme activity. NOX1 and 2 are normally activated after cell stimulation by specific agonists (e.g. G-protein coupled receptor agonists such as angiotensin II, growth factors, cytokines and mechanical forces), which induce the association of regulatory subunits and activation of the enzyme. In the case of NOX2, the cytosolic regulatory subunits are p47phox, p67phox, p40phox and Rac1 whereas NOX1 binds to the analogues of p47phox and p67phox termed NOXO1 and NOXA1, respectively, as well as Rac1. In marked contrast, NOX4 is constitutively active and does not require association with regulatory subunits, with regulation thought to occur mainly by changes in expression level. Furthermore, recent findings from several laboratories intriguingly suggest that NOX4 predominantly generates H2O2 in contrast to NOX1 and 2, which generate O2 ·− [21, 83, 103]. The biochemical basis for this property of NOX4 has recently been suggested to be related to the structure of its third extracytosolic loop, which differs significantly from those of NOX1 or 2 and contains a highly conserved histidine moiety which may prevent superoxide release or may provide protons for superoxide dismutation [108]. NOX5 is regulated by calcium binding as a consequence of EF motifs in its N-terminal domain.
NOX1 is expressed mainly in vascular smooth muscle cells (VSMC) although endothelial cell expression has also been reported. NOX2 (also known as gp91phox oxidase) was the first NADPH oxidase to be identified, being responsible for the phagocytic oxidative burst of neutrophils. Its neutrophil activity is important for non-specific host defence against microbial organisms and deficient activity of the oxidase results in chronic granulomatous disease (CGD), a condition in which affected children develop recurrent infections and inflammation. NOX2 has subsequently been found to be expressed at lower level in other inflammatory cells as well as in endothelial cells, cardiomyocytes, fibroblasts and VSMC. NOX4 is reported to be expressed in all cardiovascular cell types although its in vivo level of expression in healthy cardiovascular tissues remains to be precisely defined. NOX5 is reportedly expressed in human endothelial cells and VSMC although the data supporting its involvement in cardiovascular pathology are much weaker than that for the other NOX isoforms.
Hypertension
NOXs are expressed in all layers of the vessel wall as well as in other key organs, such as the kidney and the central nervous system (CNS), that affect the regulation of blood pressure (BP) [7, 52, 68, 94].
At the level of the vessel wall, ROS may affect vascular resistance either by direct modulation of vessel tone or by affecting vascular remodelling [92]. Vascular O2 ·− production can disrupt physiological NO signalling through its inactivation and the production of peroxynitrite, resulting in a net vasoconstrictor effect. However, H2O2 has vasodilator actions through several mechanisms and could potentially reduce vessel tone. NOX-modulated alterations in activation of redox-sensitive signalling pathways that are involved in the vascular remodelling characteristic of chronic hypertension are also important [10]. Recently, evidence was reported that NOX2 activation in inflammatory cells (in particular, T-cells) that infiltrate the vessel wall is important in angiotensin II-dependent hypertension [43].
At renal level, ROS-modulated mechanisms have been shown to operate downstream of major determinants of blood pressure, namely hormonal regulation and sodium balance. Angiotensin II-mediated experimental hypertension is associated with oxidative stress [97] and ameliorated by treatment with superoxide dismutase [71]. Angiotensin II infusion is associated with an upregulation of NADPH oxidase activity in the renal cortex in rat and rabbit models [17, 78, 116, 117], with upregulation of NOX1 mRNA expression and downregulation of NOX4 mRNA expression [17]. In contrast, a high salt intake suppresses the renin–angiotensin–aldosterone axis and yet is also associated with an increase in NADPH oxidase activity in the renal cortex, accompanied by upregulation of gp91 (NOX2) and p47phox mRNA [63]. A low salt intake produces the opposite changes. Hence there is a distinct pattern of activation of NADPH oxidase isoforms in the kidney in response to these different stimuli which are of known importance in the long-term control of blood pressure.
Finally, at CNS level, ROS are involved in modulating neuronal outputs that affect the central control of BP. Angiotensin II in the circulation is detected by certain areas of the brain outside the blood–brain barrier, including the subfornical organ (SFO). Studies by the group of Davisson showed that ROS appear to be responsible for mediating key central actions of Angiotensin II, including effects on blood pressure and thirst [125, 126]. Using adenoviral-based inhibition or overexpression of Rac1, this group also demonstrated that a Rac1-dependent NADPH oxidase (presumably NOX2) is the critical source for this ROS [124].
As suggested by the data presented above, the influence of NOXs on blood pressure has been most extensively studied in the models of angiotensin II-induced hypertension. In rodents, angiotensin II increases NOX-dependent ROS production in vessels, kidneys and CNS [17, 19, 33, 67, 77, 97, 115, 125]. This is associated with increases in NOX subunit expression and activity in all cell types within the vessel wall. Studies using systemic infusion of the peptide gp91-dstat (which inhibits activation of NOX2 and possibly other NOX isoforms) showed a significant attenuation of angiotensin II-induced hypertension and superoxide production in mice [99]. Use of siRNA targeted against p22phox in rats was reported to reduce renal cortex NOX1, 2 and 4 expression and overall renal NADPH oxidase activity and to significantly reduce the pressor response to angiotensin II [87]. Additional data derive from the studies in gene-modified mouse models. Mice with a VSMC-specific overexpression of p22phox did not develop hypertension, although interpretation of the results was potentially confounded by concurrent major changes in the expression of antioxidant genes and NO synthase [70]. Mice lacking the organizer subunit p47phox showed markedly blunted pressor responses to angiotensin II [67]. This effect could involve NOX1 or NOX2, both of which may be affected by p47phox deficiency. Indeed, mice with a global knockout of NOX1 showed significant attenuation of pressor responses to angiotensin II infusion [22, 36, 84], whereas mice overexpressing NOX1 in VSMC showed exaggerated BP responses compared to wild-type mice [22, 84]. In contrast, mice with a global deficiency of NOX2 were reported to have an unaltered BP response to angiotensin II [116], suggesting that this isoform is less important. Similarly, crossing a global NOX2 knockout with mice that had chronic angiotensin II-dependent hypertension due to transgenic liver expression of human renin failed to affect BP [111]. While NOX4 levels are known to be increased in models of renin-angiotensin II-dependent hypertension [119], its possible role in the genesis of the hypertension has been unclear. Recent studies in a model of endothelial-specific NOX4 overexpression showed that NOX4 enhanced endothelium-dependent relaxation as a result of H2O2-dependent vasodilatation [98], opposite to the effects of NOX1 or NOX2. This effect involved H2O2-dependent hyperpolarization rather than a change in NO bioactivity. In addition to the enhanced vasodilatation, NOX4 transgenic mice had a lower BP than wild-type littermates and displayed a blunted hypertensive response to angiotensin II infusion [98]. These data suggest that elevation of NOX4 could act to counteract the hypertensive effects of angiotensin II and thereby act in opposition to the pro-hypertensive effects of NOX1 and NOX2 (Fig. 2).
Contrasting vascular effects of NOX1/2 versus NOX4. a NOX1 and NOX2 promote endothelial dysfunction and vascular remodelling, at least in part through O2 ·−-dependent inactivation of NO. NOX4 on the other hand enhances endothelial-dependent relaxation through H2O2-dependent hyperpolarization. These effects may contribute to changes in BP. b Effects of endothelial-specific NOX4 overexpression on ambulatory BP measured by telemetry (top) and acetylcholine (Ach)-induced relaxation in isolated aortic rings. Tg transgenic mice; SBP systolic BP; DBP diastolic BP. ***P < 0.001. Reproduced with permission from [98]
The roles of NOX2 and NOX4 in the CNS have also been investigated. Studies using adenoviral-mediated knockdown of NOX2 or NOX4 in the subfornical organ in the CNS reported that both isoforms are required for the full pressor response to angiotensin II in the brain but that only NOX2 was coupled to the water intake response [94]. Data on the possible involvement of NOXs in other forms of experimental hypertension are largely restricted to correlative data although it was reported that norepinephrine-induced hypertension was unaffected in NOX1 knockout mice, in contrast to angiotensin II-induced hypertension [37].
In human studies, an association between ‘essential’ (i.e. idiopathic) hypertension and increased vascular NOX expression has been demonstrated in work using resistance arteries from gluteal fat biopsies [112]. Patients with hypertension have also been shown to have endothelial dysfunction in saphenous veins that is attributable to increased NOX-dependent ROS generation [44]. Human genetic studies also revealed polymorphisms of p22phox which appear relevant to hypertension. The C242T, −930(A/G) and −675(A/T) polymorphisms of CYBA, the gene encoding for p22phox, were reported to be associated with increased phagocytic NADPH oxidase activity and increased susceptibility to hypertension [88, 89, 121].
Atherosclerosis
There are several reasons to suspect an involvement of NADPH oxidases in atherosclerosis. Besides being found in all layers of the blood vessel wall [40, 44], the enzyme is present in the macrophage, a central player in atherogenesis [20]. O2 ·− can oxidize LDL and atherogenic oxidized LDL itself stimulates NADPH oxidase [3, 5, 100]. O2 ·−-mediated inactivation of NO promotes endothelial activation and VSMC proliferation, also important steps in atherogenesis [35, 59]. In addition, NOX-modulated redox signalling may accentuate both these processes. Interestingly, the propensity of atherosclerotic lesions to form in areas of turbulence such as arterial branch points might also involve mechanical activation of NOXs [38].
Mice with global NOX2 deficiency that were crossed with atherosclerosis-prone apoE −/− mice and fed a high fat diet were found to have no obvious protection against atherosclerosis when examining aortic sinus sections [62]. Similar results were reported in global p47phox knockout mice crossed with ApoE−/− mice [51]. In contrast, Barry-Lane et al. [6] found that total aortic lesion area (from arch to bifurcation) was reduced in p47phox knockout mice crossed with ApoE−/−, suggesting that NOX1 and/or NOX2 may be involved in atherogenesis. Very recently, similar observations have been described in the NOX2/ApoE double knockout mouse with a high-fat diet. [58]. Absence of NOX2 activity did not alter the disease burden within the aortic sinus but was associated with a 50% reduction in lesion area in the region between aortic arch and (iliac) bifurcation. The relevant cellular source was not delineated in these experiments but has been addressed in other work. Khatri et al. [60] employed a ligation-induced model of carotid atheroma in mice with transgenic VSMC-specific overexpression of p22phox. These animals showed enhanced growth of arterial lesions associated with a greater degree of expansive arterial remodelling, possibly a consequence of increased matrix metalloproteinase (MMP) activity. These lesions also showed enhanced angiogenesis (discussed later). Equivalent studies using genetically modified animals for the other vascular isoforms, NOX1 and NOX4, are not presently available.
Very recently, data implicating a role for NADPH oxidase in macrophage trapping in the neointima of lesions have become available. Oxidized LDL-induced changes in macrophage function via CD36 signalling were found to involve ROS-dependent inactivation of the phosphatase SHP2 [93]. This was inhibited by DPI and apocynin, suggesting that it may be NADPH oxidase-dependent although clearly not definitive.
Other indirect evidence comes from the actions of the renin inhibitor aliskiren which was found to reduce aortic sinus plaque area in ApoE−/− mice, seemingly independent of its blood pressure lowering effects, whilst also reducing vascular NOX activity [96]. In contrast, CB-1 cannabinoid receptor inhibition did not reduce atherosclerosis in ApoE−/− mice but did improve aortic endothelial-dependent vasodilation whilst reducing ROS production and NOX activity [109].
In humans, the C242T polymorphism of the CYBA gene (which encodes p22phox) has been linked to increased risk of coronary artery disease or progression of coronary artery disease in certain subgroups with the T allele [14, 15], although other authors have suggested a protective effect of this allele [29, 55]. These disparate findings may relate, at least in part, to differences in the populations under study and the endpoints used.
Human pathological specimens have also been used for the analysis of NADPH oxidase expression. Azumi et al. [4] found increased immunostaining for p22phox protein in atherosclerotic coronary arteries compared to non-atherosclerotic arteries, and showed that this co-localized with markers for endothelial cells, smooth muscle cells, infiltrating macrophages and adventitial fibroblasts. Later work by Sorescu and colleagues [106] supported these findings and found that p22phox co-localized with NOX2 predominantly in macrophages and particularly in the plaque shoulder regions. mRNA levels for p22phox and NOX2 correlated with lesion severity in this study. In contrast, NOX4 staining was most marked in the vessel media and correlated with VSMC cell density in atherosclerotic lesions. Recently, attention has also focussed upon vascular NOX5 [42, 102]. Guzik et al. studied human coronary arteries from explanted heart tissue and reported that NOX5 mRNA and protein levels were upregulated in atherosclerotic arteries, with staining evident in both the neointima and adjacent medial VSMC. NOX5 contributed significantly to overall NADPH oxidase-generated ROS in these arteries. An unexpected finding from other recent work is that NOX5 may directly activate eNOS, possibly counteracting the effects of NOX’s primary product in consuming NO [123].
Ischaemic neovascularization
A significant body of data implicates ROS in the process of angiogenesis and ischaemic neovascularisation, with in vivo evidence suggesting an involvement of NOXs in this process. Using an in vivo sponge implant model in which topical injection of VEGF or sphingosine 1-phosphate stimulated new vessel formation, Ushio-Fukai et al. [114] found that NOX2 knockout mice had reduced VEGF-induced new vessel formation. Later work used a hindlimb ligation model and found that NOX2 knockout mice developed reduced neovascularisation after ischaemia [110]. The ischaemic regions of wild-type mice subjected to hindlimb ischaemia demonstrated an increase in NOX2 protein expression and O2 ·− production, with NOX2 localized to infiltrating leucocytes acutely and then in newly formed capillaries at a later stage [110]. Supportive work comes from a retinal neovascularization model in which ROS generation in the ischaemic retina was associated with an increase in NOX2 levels and was reduced by pre-treatment with gp91ds-tat [1].
Recent work investigated the possible contribution of bone marrow-derived cells, using chimeric mice in which either bone marrow cells and/or resident tissue cells lacked NOX2 [113]. In the setting of hindlimb ischaemia, these studies showed that bone marrow-derived cells were the main source of NOX2 that was involved in post-ischaemic neovascularisation. Ebrahimian and colleagues [27] also studied bone marrow chimeric mice in a similar model but this time with or without diabetes. These authors found that NOX2 was required for post-ischaemic neovascularisation in non-diabetic mice whereas NOX2 impaired neovascularisation in diabetic mice. The authors proposed that the effects of ROS on neovascularisation depended upon the level of ROS that were generated, with high ROS levels in the setting of diabetes being detrimental. The in vivo effects of other NOX isoforms remain to be established using specific genetic approaches.
Cardiac hypertrophy and fibrosis
Myocardial hypertrophy occurs in response to chronic stresses such as cardiac overload and involves both mechanical and various neurohumoral stimuli (e.g. activation of the renin-angiotensin system, β-adrenergic activation, cytokines). NOX enzymes are found in multiple cardiac cell types—namely cardiomyocytes, endothelial cells, fibroblasts and vascular smooth muscle cells—as well as in infiltrating inflammatory cells. Furthermore, NOX enzymes, particularly NOX1 and 2, are activated by known pro-hypertrophic stimuli such as angiotensin II, tumour necrosis factor α (TNFα) and mechanical stretch [32, 53, 75, 76, 120]. It is, therefore, perhaps not unexpected that they are involved in mediating cardiac hypertrophy.
Using a model of experimental cardiac hypertrophy induced by chronic subpressor infusion of angiotensin II, Bendall et al. [9] made the initial observation that NOX2 was critical for this response since hypertrophy was markedly inhibited in NOX2 knockout mice. This was accompanied by an inhibition of interstitial cardiac fibrosis in NOX2 knockout mice. Later studies provided evidence that this effect on hypertrophy involved cardiomyocyte NOX2. Satoh and colleagues [101] studied mice with a cardiomyocyte-specific deletion of Rac1 and found that these animals had markedly blunted hypertrophic responses to angiotensin II infusion. As discussed above, Rac1 is required for NOX2 activation and these authors showed the Rac1 deficient mice also had deficient activation of NOX2 together with reduced activation of ASK1 and NF-κB.
Early studies in experimental models of aortic constriction-induced pressure overload hypertrophy found a progressive increase in left ventricular NOX-dependent ROS generation which was associated with an increase in expression of NOX2 and related oxidase subunits and was accompanied by MAP kinase (MAPK) activation—suggesting that these may be downstream targets of NOX-generated ROS [74]. However, studies in NOX2 knockout mice found that this subunit was not essential for the development of hypertrophy. These animals developed a similar degree of hypertrophy and similar increases in molecular markers of hypertrophy as wild-type littermates [13]. Similar results were obtained by an independent group [86], indicating that there are significant differences between angiotensin II and pressure overload-induced hypertrophy with respect to an essential requirement for NOX2.
Despite the lack of requirement for NOX2 for the development of pressure overload hypertrophy, it was found that NOX2 knockout mice developed less interstitial fibrosis and contractile dysfunction than wild-type littermates [41]—indicating a dissociation between hypertrophy per se and fibrosis or contractile dysfunction. In line with this, NOX2 has been shown to be critical for the development of interstitial cardiac fibrosis in models of high-dose angiotensin II infusion [57] or activation [111] as well as in aldosterone/salt hypertension [57], even though it had no effect on the extent of hypertrophy. These results may indicate cell type-specific effects of NOX2 in the development of cardiac hypertrophy and fibrosis.
In early studies of the role of NOX2 in pressure overload hypertrophy, Byrne et al. [13] found that NADPH oxidase activity increased to similar levels in NOX2 knockout and wild-type mice, and that this was the result of an increase in NOX4 levels. It was accordingly speculated that NOX4 might compensate for NOX2 in this setting. However, as mentioned earlier, the two NOX isoforms differ significantly in terms of their regulation. In addition, whereas activated NOX2 is located at the sarcolemma [49], NOX4 is found in an intracellular perinuclear location [122]. Recent studies from our laboratory have investigated the role of NOX4 using newly generated mouse models with a deletion of NOX4 or a cardiomyocyte-targeted increase in NOX4 expression. Neither model developed significant basal changes in cardiac hypertrophy or function [122]. After imposition of chronic pressure overload, however, NOX4 KO mice developed significantly greater hypertrophy and contractile dysfunction than controls whereas NOX4 transgenic mice exhibited protection against hypertrophy and failure. The underlying mechanism was found to be a NOX4-dependent preservation of myocardial capillary density during pressure overload, which involved an augmentation of stress-induced cardiomyocyte hypoxia-inducible factor 1 (HIF1) activation and the release of VEGF, resulting in increased paracrine angiogenic activity. Whereas an insufficient increase in capillary number relative to increase in cardiomyocyte size during hypertrophy is thought to promote pathological remodelling of the heart [104], the NOX4-dependent preservation of capillary density may act to counteract this (Fig. 3). These results on NOX4 along with the previous studies on NOX2 indicate that the two isoforms exert markedly different effects during pressure overload-induced cardiac hypertrophy, with NOX2 being detrimental and NOX4 beneficial.
Protective role of NOX4 during pressure overload cardiac hypertrophy. a Representative images from NOX4 knockout mice or wild-type littermates (WT) subjected to pressure overload by aortic constriction. Panel A shows whole heart sections (scale bar 2 mm); panel B shows myocardial sections stained with wheat-germ agglutinin to show cardiomyocyte area; panel C shows myocardial sections stained with Picrosirius Red to show fibrosis (scale bars 20 μm). NOX4 KO mice developed greater hypertrophy, fibrosis and remodelling than WT after pressure overload. Reproduced with permission from Zhang et al. [122]. b Schematic illustrating the mechanism underlying protective effects of NOX4. Upregulation of NOX4 enhances HIF1 activation and the release of VEGF which exerts paracrine effects to promote preservation of myocardial capillary density
Myocardial infarction, post-MI remodelling and heart failure
Post-MI cardiac remodelling refers to structural and functional alterations that occur in response to MI and predispose to the development of heart failure [95]. Features of post-MI cardiac remodelling include infarct expansion, hypertrophy of non-infarcted myocardium, ventricular dilatation, a change in chamber shape to a more spherical geometry, and systolic and diastolic dysfunction. This is associated with an increase in interstitial and perivascular fibrosis, other alterations in the extracellular matrix, and cardiomyocyte hypertrophy and apoptosis [107].
Although some of the determinants of post-MI myocardial remodelling, such as infarct size and neurohormonal status [18, 24, 45, 46], are the targets of currently available therapies, there is still a need for new therapeutic targets. Oxidative stress is implicated in the development of post-MI remodelling and could, therefore, be a therapeutic target [48, 64]. Indeed, various antioxidant agents have been shown to ameliorate remodelling after MI in rodents [61, 90], while genetically modified mice with increased activity of endogenous antioxidant systems were also protected against post-MI ventricular dilatation and dysfunction [105].
Specific involvement of NOX enzymes is suggested by clinical data demonstrating an upregulation of NOX2 in cardiomyocytes in patients who had died after MI [65]. An increase in the expression of p22phox and NOX2 proteins has also been found in the infarcted region of rats after experimental myocardial infarction [34]. More direct evidence was derived from studies in genetically altered mice. Early work showed that acute infarct size after in vivo myocardial ischaemia/reperfusion was not significantly different between NOX2 knockout mice and wild type [50]. However, studies by Bell and colleagues [8] reported that NOX2 mediates ischaemic cardiac preconditioning in isolated hearts, suggesting that it could affect infarct size under some circumstances. Later studies have investigated the role of NOX2 in post-MI remodelling. Looi and colleagues [81] studied NOX2 knockout mice subjected to permanent left coronary ligation and found that post-MI remodelling at 4 weeks was significantly reduced despite no difference in infarct size (Fig. 4). The improvement in left ventricular volumes and function was accompanied by a reduction in cardiomyocyte hypertrophy, apoptosis and interstitial fibrosis in the non-infarcted myocardium along with a decrease in mRNA levels of connective tissue growth factor (CTGF), procollagen I and ANF and lower MMP activity. Strong support for such a role of NOX2 comes from independent studies that examined post-MI remodelling in p47phox knockout mice [23]. These animals (which lack NOX2 activity) were also protected against post-MI remodelling and dysfunction with a similar pattern of underlying changes as found by Looi and colleagues. In contrast to these studies, Frantz et al. [31] failed to find a similar beneficial effect of NOX2 and instead reported a higher mortality in NOX2 knockout mice. Some caveats to this last study’s design are pertinent, e.g. the lack of littermate controls which can be a confounding factor [39], and the absence of a sham-operated group. Overall, NOX2 appears to play an important role in post-MI adverse cardiac remodelling.
Effect of NOX2 deletion on post-MI remodelling. Representative M-mode echocardiographic images from NOX2 knockout mice or wild-type littermates (WT) subjected to left coronary ligation or sham ligation surgery. The extent of LV dilatation and reduction in fractional shortening 4 weeks after MI is significantly reduced in the NOX2 knockout group compared to WT. Reproduced with permission from Looi et al. [81]
Very recently, NOXs within the central nervous system have been shown to have important effects on post-MI cardiac remodelling. It has been known for some time that superoxide production within certain nuclei of the brain (including the paraventricular nucleus) is implicated in mediating the sympathetic activation that occurs after MI and which predisposes to heart failure [73, 79]. Injection into the cerebral ventricles of superoxide dismutase (in a viral carrier) led to reduced activation of neurons in these CNS nuclei after MI [79], and resulted in reduced apoptosis and improved cardiac function and survival post-MI [80]. Recent studies showed that NOX4 was the most highly expressed NOX isoform within the paraventricular nucleus (PVN) after experimental murine MI and that siRNA-mediated knockdown of this isoform in the PVN reduced sympathetic outflow from the brain, reduced peri-infarct apoptosis and improved cardiac performance [54].
The main adverse clinical consequence of cardiac remodelling is the development of overt heart failure. Clinical data are available regarding NADPH oxidases in end-stage heart failure, mostly derived from explanted failing hearts at the time of transplantation. Our group demonstrated that NADPH oxidase activity was significantly upregulated in such hearts compared to non-failing hearts and that this was associated with a greater membrane translocation of p47phox [49]. Similar findings were made by Maack and colleagues [82], who also described increased Rac1 translocation from cytosol to membrane, associated with elevated rac1-GTPase activity. Downstream pathways have also been examined in this context and there is evidence of increased activation of ERK1/2 and p38MAPK in parallel with increased NOX activity in end-stage failing hearts, both in the left and right ventricles [91]. It has been suggested that the right ventricle may be more susceptible to increased oxidative stress due to poorer antioxidant defence activation and that this may be relevant in the development of overt heart failure [11]. An increase in NOX2-dependent ROS generation associated with endothelial dysfunction has also been reported in saphenous veins obtained from patients with heart failure [26], suggesting that increased NOX2 activation may have wider systemic effects in the setting of heart failure. In an experimental model of diastolic heart failure, use of a pharmacological agent to enhance eNOS activity produced attenuation of diastolic dysfunction and reduced levels of NOX2 subunits, suggesting a role for oxidative stress in modulating cardiac function in this setting too [118].
Conclusions
NADPH oxidases are major sources of ROS in the heart and vasculature that appear to have an important role in the pathogenesis of several cardiovascular conditions. While clinical evidence for their importance is largely correlative, more robust data derive from experiments in gene-modified animals. These studies have largely focused on NOX2 and NOX1, whereas the roles of NOX4 have been less clear. Work to date indicates that NOX1 and/or 2-mediated signalling may be detrimental in hypertension, atherosclerosis, cardiac hypertrophy and remodelling. However, recent work suggests that NOX4 may play a protective role in the heart subjected to chronic pressure overload as well as in the vasculature in the setting of hypertension. Additional research on the isoform-specific roles of the NOXs and on the specific downstream targets that they affect may define suitable therapeutic targets for cardiovascular diseases.
References
Al-Shabrawey M, Bartoli M, El-Remessy AB, Platt DH, Matragoon S, Behzadian MA, Caldwell RW, Caldwell RB (2005) Inhibition of NAD(P)H oxidase activity blocks vascular endothelial growth factor overexpression and neovascularization during ischemic retinopathy. Am J Pathol 167(2):599–607. 167/2/599[pii]
Aldieri E, Riganti C, Polimeni M, Gazzano E, Lussiana C, Campia I, Ghigo D (2008) Classical inhibitors of NOX NAD(P)H oxidases are not specific. Curr Drug Metab 9(8):686–696
Aviram M, Rosenblat M, Etzioni A, Levy R (1996) Activation of NADPH oxidase required for macrophage-mediated oxidation of low-density lipoprotein. Metabolism 45(9):1069–1079
Azumi H, Inoue N, Takeshita S, Rikitake Y, Kawashima S, Hayashi Y, Itoh H, Yokoyama M (1999) Expression of NADH/NADPH oxidase p22phox in human coronary arteries. Circulation 100(14):1494–1498
Bae YS, Lee JH, Choi SH, Kim S, Almazan F, Witztum JL, Miller YI (2009) Macrophages generate reactive oxygen species in response to minimally oxidized low-density lipoprotein: toll-like receptor 4- and spleen tyrosine kinase-dependent activation of NADPH oxidase 2. Circ Res 104(2):210–218. doi:10.1161/CIRCRESAHA.108.181040, 221p following 218
Barry-Lane PA, Patterson C, van der Merwe M, Hu Z, Holland SM, Yeh ET, Runge MS (2001) p47phox is required for atherosclerotic lesion progression in ApoE(−/−) mice. J Clin Invest 108(10):1513–1522. doi:10.1172/JCI11927
Bedard K, Krause KH (2007) The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87(1):245–313. doi:10.1152/physrev.00044.2005
Bell RM, Cave AC, Johar S, Hearse DJ, Shah AM, Shattock MJ (2005) Pivotal role of NOX-2-containing NADPH oxidase in early ischemic preconditioning. Faseb J 19(14):2037–2039
Bendall JK, Cave AC, Heymes C, Gall N, Shah AM (2002) Pivotal role of a gp91(phox)-containing NADPH oxidase in angiotensin II-induced cardiac hypertrophy in mice. Circulation 105(3):293–296
Bleeke T, Zhang H, Madamanchi N, Patterson C, Faber JE (2004) Catecholamine-induced vascular wall growth is dependent on generation of reactive oxygen species. Circ Res 94(1):37–45. doi:10.1161/01.RES.0000109412.80157.7D
Borchi E, Bargelli V, Stillitano F, Giordano C, Sebastiani M, Nassi PA, d’Amati G, Cerbai E, Nediani C (2010) Enhanced ROS production by NADPH oxidase is correlated to changes in antioxidant enzyme activity in human heart failure. Biochim Biophys Acta 1802(3):331–338
Brandes RP, Weissmann N, Schroder K (2010) NADPH oxidases in cardiovascular disease. Free Radic Biol Med 49(5):687–706
Byrne JA, Grieve DJ, Bendall JK, Li JM, Gove C, Lambeth JD, Cave AC, Shah AM (2003) Contrasting roles of NADPH oxidase isoforms in pressure-overload versus angiotensin II-induced cardiac hypertrophy. Circ Res 93(9):802–805. doi:10.1161/01.RES.0000099504.30207.F5
Cahilly C, Ballantyne CM, Lim DS, Gotto A, Marian AJ (2000) A variant of p22(phox), involved in generation of reactive oxygen species in the vessel wall, is associated with progression of coronary atherosclerosis. Circ Res 86(4):391–395
Cai H, Duarte N, Wilcken DE, Wang XL (1999) NADH/NADPH oxidase p22 phox C242T polymorphism and coronary artery disease in the Australian population. Eur J Clin Invest 29(9):744–748. eci531[pii]
Cave AC, Brewer AC, Narayanapanicker A, Ray R, Grieve DJ, Walker S, Shah AM (2006) NADPH oxidases in cardiovascular health and disease. Antioxid Redox Signal 8(5–6):691–728. doi:10.1089/ars.2006.8.691
Chabrashvili T, Kitiyakara C, Blau J, Karber A, Aslam S, Welch WJ, Wilcox CS (2003) Effects of ANG II type 1 and 2 receptors on oxidative stress, renal NADPH oxidase, and SOD expression. Am J Physiol Regul Integr Comp Physiol 285(1):R117–R124
Chareonthaitawee P, Christian TF, Hirose K, Gibbons RJ, Rumberger JA (1995) Relation of initial infarct size to extent of left ventricular remodeling in the year after acute myocardial infarction. J Am Coll Cardiol 25(3):567–573. doi:10.1016/0735-1097(94)00431-O
Cifuentes ME, Rey FE, Carretero OA, Pagano PJ (2000) Upregulation of p67(phox) and gp91(phox) in aortas from angiotensin II-infused mice. Am J Physiol Heart Circ Physiol 279(5):H2234–H2240
Dale DC, Boxer L, Liles WC (2008) The phagocytes: neutrophils and monocytes. Blood 112(4):935–945. doi:10.1182/blood-2007-12-077917
Dikalov SI, Dikalova AE, Bikineyeva AT, Schmidt HH, Harrison DG, Griendling KK (2008) Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic Biol Med 45(9):1340–1351
Dikalova A, Clempus R, Lassegue B, Cheng G, McCoy J, Dikalov S, San Martin A, Lyle A, Weber DS, Weiss D, Taylor WR, Schmidt HH, Owens GK, Lambeth JD, Griendling KK (2005) Nox1 overexpression potentiates angiotensin II-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation 112(17):2668–2676. doi:10.1161/CIRCULATIONAHA.105.538934
Doerries C, Grote K, Hilfiker-Kleiner D, Luchtefeld M, Schaefer A, Holland SM, Sorrentino S, Manes C, Schieffer B, Drexler H, Landmesser U (2007) Critical role of the NAD(P)H oxidase subunit p47phox for left ventricular remodeling/dysfunction and survival after myocardial infarction. Circ Res 100(6):894–903. doi:10.1161/01.RES.0000261657.76299.ff
Doughty RN, Whalley GA, Walsh HA, Gamble GD, Lopez-Sendon J, Sharpe N (2004) Effects of carvedilol on left ventricular remodeling after acute myocardial infarction: the CAPRICORN Echo Substudy. Circulation 109(2):201–206. doi:10.1161/01.CIR.0000108928.25690.94
Droge W (2002) Free radicals in the physiological control of cell function. Physiol Rev 82(1):47–95. doi:10.1152/physrev.00018.2001
Dworakowski R, Walker S, Momin A, Desai J, El-Gamel A, Wendler O, Kearney MT, Shah AM (2008) Reduced nicotinamide adenine dinucleotide phosphate oxidase-derived superoxide and vascular endothelial dysfunction in human heart failure. J Am Coll Cardiol 51(14):1349–1356
Ebrahimian TG, Heymes C, You D, Blanc-Brude O, Mees B, Waeckel L, Duriez M, Vilar J, Brandes RP, Levy BI, Shah AM, Silvestre JS (2006) NADPH oxidase-derived overproduction of reactive oxygen species impairs postischemic neovascularization in mice with type 1 diabetes. Am J Pathol 169(2):719–728. 169/2/719[pii]
Eleuteri E, Magno F, Gnemmi I, Carbone M, Colombo M, La Rocca G, Anzalone R, Genta FT, Zummo G, Di Stefano A, Giannuzzi P (2009) Role of oxidative and nitrosative stress biomarkers in chronic heart failure. Front Biosci 14:2230–2237
Fan M, Kahonen M, Rontu R, Lehtinen R, Viik J, Niemi M, Nieminen T, Niemela K, Porsti I, Koobi T, Turjanmaa V, Lehtimaki T (2006) The p22phox C242T gene polymorphism is associated with a reduced risk of angiographically verified coronary artery disease in a high-risk Finnish Caucasian population. The Finnish Cardiovascular Study. Am Heart J 152(3):538–542. doi:10.1016/j.ahj.2006.02.018
Forstermann U (2008) Oxidative stress in vascular disease: causes, defense mechanisms and potential therapies. Nat Clin Pract Cardiovasc Med 5(6):338–349
Frantz S, Brandes RP, Hu K, Rammelt K, Wolf J, Scheuermann H, Ertl G, Bauersachs J (2006) Left ventricular remodeling after myocardial infarction in mice with targeted deletion of the NADPH oxidase subunit gp91PHOX. Basic Res Cardiol 101(2):127–132. doi:10.1007/s00395-005-0568-x
Frey RS, Rahman A, Kefer JC, Minshall RD, Malik AB (2002) PKCzeta regulates TNF-alpha-induced activation of NADPH oxidase in endothelial cells. Circ Res 90(9):1012–1019
Fukui T, Ishizaka N, Rajagopalan S, Laursen JB, Capers Qt, Taylor WR, Harrison DG, de Leon H, Wilcox JN, Griendling KK (1997) p22phox mRNA expression and NADPH oxidase activity are increased in aortas from hypertensive rats. Circ Res 80(1):45–51
Fukui T, Yoshiyama M, Hanatani A, Omura T, Yoshikawa J, Abe Y (2001) Expression of p22-phox and gp91-phox, essential components of NADPH oxidase, increases after myocardial infarction. Biochem Biophys Res Commun 281(5):1200–1206. doi:10.1006/bbrc.2001.4493
Garg UC, Hassid A (1989) Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest 83(5):1774–1777. doi:10.1172/JCI114081
Gavazzi G, Banfi B, Deffert C, Fiette L, Schappi M, Herrmann F, Krause KH (2006) Decreased blood pressure in NOX1-deficient mice. FEBS Lett 580(2):497–504
Gavazzi G, Deffert C, Trocme C, Schappi M, Herrmann FR, Krause KH (2007) NOX1 deficiency protects from aortic dissection in response to angiotensin II. Hypertension 50(1):189–196
Godbole AS, Lu X, Guo X, Kassab GS (2009) NADPH oxidase has a directional response to shear stress. Am J Physiol Heart Circ Physiol 296(1):H152–H158. doi:10.1152/ajpheart.01251.2007
Gorog DA, Tanno M, Kabir AM, Kanaganayagam GS, Bassi R, Fisher SG, Marber MS (2003) Varying susceptibility to myocardial infarction among C57BL/6 mice of different genetic background. J Mol Cell Cardiol 35(6):705–708. S0022282803000828[pii]
Griendling KK (2004) Novel NAD(P)H oxidases in the cardiovascular system. Heart 90(5):491–493
Grieve DJ, Byrne JA, Siva A, Layland J, Johar S, Cave AC, Shah AM (2006) Involvement of the nicotinamide adenosine dinucleotide phosphate oxidase isoform Nox2 in cardiac contractile dysfunction occurring in response to pressure overload. J Am Coll Cardiol 47(4):817–826
Guzik TJ, Chen W, Gongora MC, Guzik B, Lob HE, Mangalat D, Hoch N, Dikalov S, Rudzinski P, Kapelak B, Sadowski J, Harrison DG (2008) Calcium-dependent NOX5 nicotinamide adenine dinucleotide phosphate oxidase contributes to vascular oxidative stress in human coronary artery disease. J Am Coll Cardiol 52(22):1803–1809. doi:10.1016/j.jacc.2008.07.063
Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG (2007) Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204(10):2449–2460
Guzik TJ, West NE, Black E, McDonald D, Ratnatunga C, Pillai R, Channon KM (2000) Vascular superoxide production by NAD(P)H oxidase: association with endothelial dysfunction and clinical risk factors. Circ Res 86(9):E85–E90
Harada K, Sugaya T, Murakami K, Yazaki Y, Komuro I (1999) Angiotensin II type 1A receptor knockout mice display less left ventricular remodeling and improved survival after myocardial infarction. Circulation 100(20):2093–2099
Hayashi M, Tsutamoto T, Wada A, Tsutsui T, Ishii C, Ohno K, Fujii M, Taniguchi A, Hamatani T, Nozato Y, Kataoka K, Morigami N, Ohnishi M, Kinoshita M, Horie M (2003) Immediate administration of mineralocorticoid receptor antagonist spironolactone prevents post-infarct left ventricular remodeling associated with suppression of a marker of myocardial collagen synthesis in patients with first anterior acute myocardial infarction. Circulation 107(20):2559–2565. doi:10.1161/01.CIR.0000068340.96506.0F
Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, Brandes RP (2008) Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 51(2):211–217. doi:10.1161/HYPERTENSIONAHA.107.100214
Heusch G, Schulz R (2011) A radical view on the contractile machinery in human heart failure. J Am Coll Cardiol 57(3):310–312
Heymes C, Bendall JK, Ratajczak P, Cave AC, Samuel JL, Hasenfuss G, Shah AM (2003) Increased myocardial NADPH oxidase activity in human heart failure. J Am Coll Cardiol 41(12):2164–2171
Hoffmeyer MR, Jones SP, Ross CR, Sharp B, Grisham MB, Laroux FS, Stalker TJ, Scalia R, Lefer DJ (2000) Myocardial ischemia/reperfusion injury in NADPH oxidase-deficient mice. Circ Res 87(9):812–817
Hsich E, Segal BH, Pagano PJ, Rey FE, Paigen B, Deleonardis J, Hoyt RF, Holland SM, Finkel T (2000) Vascular effects following homozygous disruption of p47(phox): an essential component of NADPH oxidase. Circulation 101(11):1234–1236
Hur J, Lee P, Kim MJ, Kim Y, Cho YW (2010) Ischemia-activated microglia induces neuronal injury via activation of gp91phox NADPH oxidase. Biochem Biophys Res Commun 391(3):1526–1530
Hwang J, Ing MH, Salazar A, Lassegue B, Griendling K, Navab M, Sevanian A, Hsiai TK (2003) Pulsatile versus oscillatory shear stress regulates NADPH oxidase subunit expression: implication for native LDL oxidation. Circ Res 93(12):1225–1232. doi:10.1161/01.RES.0000104087.29395.66
Infanger DW, Cao X, Butler SD, Burmeister MA, Zhou Y, Stupinski JA, Sharma RV, Davisson RL (2010) Silencing nox4 in the paraventricular nucleus improves myocardial infarction-induced cardiac dysfunction by attenuating sympathoexcitation and periinfarct apoptosis. Circ Res 106(11):1763–1774
Inoue N, Kawashima S, Kanazawa K, Yamada S, Akita H, Yokoyama M (1998) Polymorphism of the NADH/NADPH oxidase p22 phox gene in patients with coronary artery disease. Circulation 97(2):135–137
Janssen-Heininger YM, Mossman BT, Heintz NH, Forman HJ, Kalyanaraman B, Finkel T, Stamler JS, Rhee SG, van der Vliet A (2008) Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic Biol Med 45(1):1–17. doi:10.1016/j.freeradbiomed.2008.03.011
Johar S, Cave AC, Narayanapanicker A, Grieve DJ, Shah AM (2006) Aldosterone mediates angiotensin II-induced interstitial cardiac fibrosis via a Nox2-containing NADPH oxidase. FASEB J 20(9):1546–1548. doi:10.1096/fj.05-4642fje
Judkins CP, Diep H, Broughton BR, Mast AE, Hooker EU, Miller AA, Selemidis S, Dusting GJ, Sobey CG, Drummond GR (2010) Direct evidence of a role for Nox2 in superoxide production, reduced nitric oxide bioavailability, and early atherosclerotic plaque formation in ApoE−/− mice. Am J Physiol Heart Circ Physiol 298(1):H24–H32
Khan BV, Harrison DG, Olbrych MT, Alexander RW, Medford RM (1996) Nitric oxide regulates vascular cell adhesion molecule 1 gene expression and redox-sensitive transcriptional events in human vascular endothelial cells. Proc Natl Acad Sci USA 93(17):9114–9119
Khatri JJ, Johnson C, Magid R, Lessner SM, Laude KM, Dikalov SI, Harrison DG, Sung HJ, Rong Y, Galis ZS (2004) Vascular oxidant stress enhances progression and angiogenesis of experimental atheroma. Circulation 109(4):520–525. doi:10.1161/01.CIR.0000109698.70638.2B
Kinugawa S, Tsutsui H, Hayashidani S, Ide T, Suematsu N, Satoh S, Utsumi H, Takeshita A (2000) Treatment with dimethylthiourea prevents left ventricular remodeling and failure after experimental myocardial infarction in mice: role of oxidative stress. Circ Res 87(5):392–398
Kirk EA, Dinauer MC, Rosen H, Chait A, Heinecke JW, LeBoeuf RC (2000) Impaired superoxide production due to a deficiency in phagocyte NADPH oxidase fails to inhibit atherosclerosis in mice. Arterioscler Thromb Vasc Biol 20(6):1529–1535
Kitiyakara C, Chabrashvili T, Chen Y, Blau J, Karber A, Aslam S, Welch WJ, Wilcox CS (2003) Salt intake, oxidative stress, and renal expression of NADPH oxidase and superoxide dismutase. J Am Soc Nephrol 14(11):2775–2782
Kleinbongard P, Heusch G, Schulz R (2010) TNFalpha in atherosclerosis, myocardial ischemia/reperfusion and heart failure. Pharmacol Ther 127(3):295–314
Krijnen PA, Meischl C, Hack CE, Meijer CJ, Visser CA, Roos D, Niessen HW (2003) Increased Nox2 expression in human cardiomyocytes after acute myocardial infarction. J Clin Pathol 56(3):194–199
Lambeth JD (2004) NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol 4(3):181–189. 10.1038/nri1312nri1312[pii]
Landmesser U, Cai H, Dikalov S, McCann L, Hwang J, Jo H, Holland SM, Harrison DG (2002) Role of p47(phox) in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension 40(4):511–515
Lassegue B, Clempus RE (2003) Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol 285(2):R277–R297. doi:10.1152/ajpregu.00758.2002
Lassegue B, Griendling KK (2010) NADPH oxidases: functions and pathologies in the vasculature. Arterioscler Thromb Vasc Biol 30(4):653–661
Laude K, Cai H, Fink B, Hoch N, Weber DS, McCann L, Kojda G, Fukai T, Schmidt HH, Dikalov S, Ramasamy S, Gamez G, Griendling KK, Harrison DG (2005) Hemodynamic and biochemical adaptations to vascular smooth muscle overexpression of p22phox in mice. Am J Physiol Heart Circ Physiol 288(1):H7–H12. doi:10.1152/ajpheart.00637.2004
Laursen JB, Rajagopalan S, Galis Z, Tarpey M, Freeman BA, Harrison DG (1997) Role of superoxide in angiotensin II-induced but not catecholamine-induced hypertension. Circulation 95(3):588–593
Lee MY, Griendling KK (2008) Redox signaling, vascular function, and hypertension. Antioxid Redox Signal 10(6):1045–1059
Leenen FH (2007) Brain mechanisms contributing to sympathetic hyperactivity and heart failure. Circ Res 101(3):221–223
Li JM, Gall NP, Grieve DJ, Chen M, Shah AM (2002) Activation of NADPH oxidase during progression of cardiac hypertrophy to failure. Hypertension 40(4):477–484
Li JM, Mullen AM, Yun S, Wientjes F, Brouns GY, Thrasher AJ, Shah AM (2002) Essential role of the NADPH oxidase subunit p47(phox) in endothelial cell superoxide production in response to phorbol ester and tumor necrosis factor-alpha. Circ Res 90(2):143–150
Li JM, Shah AM (2003) Mechanism of endothelial cell NADPH oxidase activation by angiotensin II. Role of the p47phox subunit. J Biol Chem 278(14):12094–12100. doi:10.1074/jbc.M209793200
Li JM, Wheatcroft S, Fan LM, Kearney MT, Shah AM (2004) Opposing roles of p47phox in basal versus angiotensin II-stimulated alterations in vascular O2-production, vascular tone, and mitogen-activated protein kinase activation. Circulation 109(10):1307–1313. doi:10.1161/01.CIR.0000118463.23388.B9
Li N, Yi FX, Spurrier JL, Bobrowitz CA, Zou AP (2002) Production of superoxide through NADH oxidase in thick ascending limb of Henle’s loop in rat kidney. Am J Physiol Renal Physiol 282(6):F1111–F1119
Lindley TE, Doobay MF, Sharma RV, Davisson RL (2004) Superoxide is involved in the central nervous system activation and sympathoexcitation of myocardial infarction-induced heart failure. Circ Res 94(3):402–409
Lindley TE, Infanger DW, Rishniw M, Zhou Y, Doobay MF, Sharma RV, Davisson RL (2009) Scavenging superoxide selectively in mouse forebrain is associated with improved cardiac function and survival following myocardial infarction. Am J Physiol Regul Integr Comp Physiol 296(1):R1–R8
Looi YH, Grieve DJ, Siva A, Walker SJ, Anilkumar N, Cave AC, Marber M, Monaghan MJ, Shah AM (2008) Involvement of Nox2 NADPH oxidase in adverse cardiac remodeling after myocardial infarction. Hypertension 51(2):319–325. doi:10.1161/HYPERTENSIONAHA.107.101980
Maack C, Kartes T, Kilter H, Schafers HJ, Nickenig G, Bohm M, Laufs U (2003) Oxygen free radical release in human failing myocardium is associated with increased activity of rac1-GTPase and represents a target for statin treatment. Circulation 108(13):1567–1574
Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG (2006) Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal 18(1):69–82
Matsuno K, Yamada H, Iwata K, Jin D, Katsuyama M, Matsuki M, Takai S, Yamanishi K, Miyazaki M, Matsubara H, Yabe-Nishimura C (2005) Nox1 is involved in angiotensin II-mediated hypertension: a study in Nox1-deficient mice. Circulation 112(17):2677–2685. doi:10.1161/CIRCULATIONAHA.105.573709
Maxwell SR (2000) Coronary artery disease–free radical damage, antioxidant protection and the role of homocysteine. Basic Res Cardiol 95(Suppl 1):I65–I71
Maytin M, Siwik DA, Ito M, Xiao L, Sawyer DB, Liao R, Colucci WS (2004) Pressure overload-induced myocardial hypertrophy in mice does not require gp91phox. Circulation 109(9):1168–1171. doi:10.1161/01.CIR.0000117229.60628.2F
Modlinger P, Chabrashvili T, Gill PS, Mendonca M, Harrison DG, Griendling KK, Li M, Raggio J, Wellstein A, Chen Y, Welch WJ, Wilcox CS (2006) RNA silencing in vivo reveals role of p22phox in rat angiotensin slow pressor response. Hypertension 47(2):238–244. doi:10.1161/01.HYP.0000200023.02195.73
Moreno MU, San Jose G, Fortuno A, Beloqui O, Diez J, Zalba G (2006) The C242T CYBA polymorphism of NADPH oxidase is associated with essential hypertension. J Hypertens 24(7):1299–1306. doi:10.1097/01.hjh.0000234110.54110.56
Moreno MU, San Jose G, Fortuno A, Beloqui O, Redon J, Chaves FJ, Corella D, Diez J, Zalba G (2007) A novel CYBA variant, the -675A/T polymorphism, is associated with essential hypertension. J Hypertens 25(8):1620–1626. doi:10.1097/HJH.0b013e3281ac211d
Nakamura R, Egashira K, Machida Y, Hayashidani S, Takeya M, Utsumi H, Tsutsui H, Takeshita A (2002) Probucol attenuates left ventricular dysfunction and remodeling in tachycardia-induced heart failure: roles of oxidative stress and inflammation. Circulation 106(3):362–367
Nediani C, Borchi E, Giordano C, Baruzzo S, Ponziani V, Sebastiani M, Nassi P, Mugelli A, d’Amati G, Cerbai E (2007) NADPH oxidase-dependent redox signaling in human heart failure: relationship between the left and right ventricle. J Mol Cell Cardiol 42(4):826–834
Park Y, Yang J, Zhang H, Chen X, Zhang C (2011) Effect of PAR2 in regulating TNF-alpha and NAD(P)H oxidase in coronary arterioles in type 2 diabetic mice. Basic Res Cardiol 106(1):111–123. doi:10.1007/s00395-010-0129-9
Park YM, Febbraio M, Silverstein RL (2009) CD36 modulates migration of mouse and human macrophages in response to oxidized LDL and may contribute to macrophage trapping in the arterial intima. J Clin Invest 119(1):136–145. doi:10.1172/JCI35535
Peterson JR, Burmeister MA, Tian X, Zhou Y, Guruju MR, Stupinski JA, Sharma RV, Davisson RL (2009) Genetic silencing of Nox2 and Nox4 reveals differential roles of these NADPH oxidase homologues in the vasopressor and dipsogenic effects of brain angiotensin II. Hypertension 54(5):1106–1114
Pfeffer MA, Braunwald E (1990) Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation 81(4):1161–1172
Poss J, Werner C, Lorenz D, Gensch C, Bohm M, Laufs U (2010) The renin inhibitor aliskiren upregulates pro-angiogenic cells and reduces atherogenesis in mice. Basic Res Cardiol 105(6):725–735
Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG (1996) Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest 97(8):1916–1923
Ray R, Murdoch CE, Wang M, Santos CX, Zhang M, Alom-Ruiz S, Anilkumar N, Ouattara A, Cave AC, Walker SJ, Grieve DJ, Charles RL, Eaton P, Brewer AC, Shah AM (2011) Endothelial Nox4 NADPH Oxidase Enhances Vasodilatation and Reduces Blood Pressure In Vivo. Arterioscler Thromb Vasc Biol
Rey FE, Cifuentes ME, Kiarash A, Quinn MT, Pagano PJ (2001) Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O(2)(−) and systolic blood pressure in mice. Circ Res 89(5):408–414
Rueckschloss U, Galle J, Holtz J, Zerkowski HR, Morawietz H (2001) Induction of NAD(P)H oxidase by oxidized low-density lipoprotein in human endothelial cells: antioxidative potential of hydroxymethylglutaryl coenzyme A reductase inhibitor therapy. Circulation 104(15):1767–1772
Satoh M, Ogita H, Takeshita K, Mukai Y, Kwiatkowski DJ, Liao JK (2006) Requirement of Rac1 in the development of cardiac hypertrophy. Proc Natl Acad Sci USA 103(19):7432–7437. doi:10.1073/pnas.0510444103
Schulz E, Munzel T (2008) NOX5, a new “radical” player in human atherosclerosis? J Am Coll Cardiol 52(22):1810–1812. doi:10.1016/j.jacc.2008.08.040
Serrander L, Cartier L, Bedard K, Banfi B, Lardy B, Plastre O, Sienkiewicz A, Forro L, Schlegel W, Krause KH (2007) NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J 406(1):105–114
Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, Liao R, Colucci WS, Walsh K (2005) Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest 115(8):2108–2118
Shiomi T, Tsutsui H, Matsusaka H, Murakami K, Hayashidani S, Ikeuchi M, Wen J, Kubota T, Utsumi H, Takeshita A (2004) Overexpression of glutathione peroxidase prevents left ventricular remodeling and failure after myocardial infarction in mice. Circulation 109(4):544–549. doi:10.1161/01.CIR.0000109701.77059.E9
Sorescu D, Weiss D, Lassegue B, Clempus RE, Szocs K, Sorescu GP, Valppu L, Quinn MT, Lambeth JD, Vega JD, Taylor WR, Griendling KK (2002) Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation 105(12):1429–1435
Swynghedauw B (1999) Molecular mechanisms of myocardial remodeling. Physiol Rev 79(1):215–262
Takac I, Schroder K, Zhang L, Lardy B, Anilkumar N, Lambeth JD, Shah AM, Morel F, Brandes RP (2011) The E-loop Is Involved in Hydrogen Peroxide Formation by the NADPH Oxidase Nox4. J Biol Chem 286(15):13304–13313. doi:10.1074/jbc.M110.192138
Tiyerili V, Zimmer S, Jung S, Wassmann K, Naehle CP, Lutjohann D, Zimmer A, Nickenig G, Wassmann S (2010) CB1 receptor inhibition leads to decreased vascular AT1 receptor expression, inhibition of oxidative stress and improved endothelial function. Basic Res Cardiol 105(4):465–477
Tojo T, Ushio-Fukai M, Yamaoka-Tojo M, Ikeda S, Patrushev N, Alexander RW (2005) Role of gp91phox (Nox2)-containing NAD(P)H oxidase in angiogenesis in response to hindlimb ischemia. Circulation 111(18):2347–2355. doi:10.1161/01.CIR.0000164261.62586.14
Touyz RM, Mercure C, He Y, Javeshghani D, Yao G, Callera GE, Yogi A, Lochard N, Reudelhuber TL (2005) Angiotensin II-dependent chronic hypertension and cardiac hypertrophy are unaffected by gp91phox-containing NADPH oxidase. Hypertension 45(4):530–537. doi:10.1161/01.HYP.0000158845.49943.5e
Touyz RM, Schiffrin EL (2001) Increased generation of superoxide by angiotensin II in smooth muscle cells from resistance arteries of hypertensive patients: role of phospholipase D-dependent NAD(P)H oxidase-sensitive pathways. J Hypertens 19(7):1245–1254
Urao N, Inomata H, Razvi M, Kim HW, Wary K, McKinney R, Fukai T, Ushio-Fukai M (2008) Role of nox2-based NADPH oxidase in bone marrow and progenitor cell function involved in neovascularization induced by hindlimb ischemia. Circ Res 103(2):212–220. doi:10.1161/CIRCRESAHA.108.176230
Ushio-Fukai M, Tang Y, Fukai T, Dikalov SI, Ma Y, Fujimoto M, Quinn MT, Pagano PJ, Johnson C, Alexander RW (2002) Novel role of gp91(phox)-containing NAD(P)H oxidase in vascular endothelial growth factor-induced signaling and angiogenesis. Circ Res 91(12):1160–1167
Virdis A, Neves MF, Amiri F, Touyz RM, Schiffrin EL (2004) Role of NAD(P)H oxidase on vascular alterations in angiotensin II-infused mice. J Hypertens 22(3):535–542. 00004872-200403000-00016[pii]
Wang HD, Xu S, Johns DG, Du Y, Quinn MT, Cayatte AJ, Cohen RA (2001) Role of NADPH oxidase in the vascular hypertrophic and oxidative stress response to angiotensin II in mice. Circ Res 88(9):947–953
Welch WJ, Blau J, Xie H, Chabrashvili T, Wilcox CS (2005) Angiotensin-induced defects in renal oxygenation: role of oxidative stress. Am J Physiol Heart Circ Physiol 288(1):H22–H28
Westermann D, Riad A, Richter U, Jager S, Savvatis K, Schuchardt M, Bergmann N, Tolle M, Nagorsen D, Gotthardt M, Schultheiss HP, Tschope C (2009) Enhancement of the endothelial NO synthase attenuates experimental diastolic heart failure. Basic Res Cardiol 104(5):499–509
Wingler K, Wunsch S, Kreutz R, Rothermund L, Paul M, Schmidt HH (2001) Upregulation of the vascular NAD(P)H-oxidase isoforms Nox1 and Nox4 by the renin-angiotensin system in vitro and in vivo. Free Radic Biol Med 31(11):1456–1464. S0891584901007274[pii]
Xiao L, Pimentel DR, Wang J, Singh K, Colucci WS, Sawyer DB (2002) Role of reactive oxygen species and NAD(P)H oxidase in alpha(1)-adrenoceptor signaling in adult rat cardiac myocytes. Am J Physiol Cell Physiol 282(4):C926–C934. doi:10.1152/ajpcell.00254.2001
Zalba G, San Jose G, Moreno MU, Fortuno A, Diez J (2005) NADPH oxidase-mediated oxidative stress: genetic studies of the p22(phox) gene in hypertension. Antioxid Redox Signal 7(9–10):1327–1336. doi:10.1089/ars.2005.7.1327
Zhang M, Brewer AC, Schroder K, Santos CX, Grieve DJ, Wang M, Anilkumar N, Yu B, Dong X, Walker SJ, Brandes RP, Shah AM (2010) NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proc Natl Acad Sci USA 107(42):18121–18126
Zhang Q, Malik P, Pandey D, Gupta S, Jagnandan D, Belin de Chantemele E, Banfi B, Marrero MB, Rudic RD, Stepp DW, Fulton DJ (2008) Paradoxical activation of endothelial nitric oxide synthase by NADPH oxidase. Arterioscler Thromb Vasc Biol 28(9):1627–1633. doi:10.1161/ATVBAHA.108.168278
Zimmerman MC, Dunlay RP, Lazartigues E, Zhang Y, Sharma RV, Engelhardt JF, Davisson RL (2004) Requirement for Rac1-dependent NADPH oxidase in the cardiovascular and dipsogenic actions of angiotensin II in the brain. Circ Res 95(5):532–539
Zimmerman MC, Lazartigues E, Lang JA, Sinnayah P, Ahmad IM, Spitz DR, Davisson RL (2002) Superoxide mediates the actions of angiotensin II in the central nervous system. Circ Res 91(11):1038–1045
Zimmerman MC, Lazartigues E, Sharma RV, Davisson RL (2004) Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. Circ Res 95(2):210–216
Acknowledgments
The authors’ work is supported by the British Heart Foundation (RG/08/011/25922, CH/99001; RE/08/003); a Leducq Foundation Transatlantic Network of Excellence Award; EU FP6 grant LSHM-CT-2005-018833 (EUGeneHeart); the British Cardiovascular Society; and the National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre award to Guy’s & St Thomas’ NHS Foundation Trust in partnership with King’s College London and King’s College Hospital NHS Foundation Trust.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Sirker, A., Zhang, M. & Shah, A.M. NADPH oxidases in cardiovascular disease: insights from in vivo models and clinical studies. Basic Res Cardiol 106, 735–747 (2011). https://doi.org/10.1007/s00395-011-0190-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00395-011-0190-z