Abstract
Aim
To evaluate the preventive and therapeutic effects of Lactobacillus casei Zhang on impaired glucose tolerance (IGT) by using fructose-induced hyperinsulinemia rats.
Methods
Rats were fed 25 % fructose solution for hyperinsulinemia with L. casei Zhang for prevention or therapy. Serum levels of insulin, glucagon-like peptide-2 (GLP-2), osteocalcin, malondialdehyde (MDA), total intestinal bile acids and hepatic glycogen contents were determined by assay kits. The major bacteria from feces and liver expression of adiponectin receptor 2 (AdipoR2), liver X receptor-α (LXR-α), peroxisome proliferator-activated receptor gamma (PPAR-γ) and vitamin K epoxide reductase complex subunit 1 mRNA were assessed by RT-PCR. Pancreas injury was evaluated by histological analysis.
Results
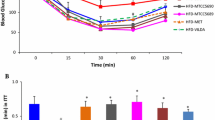
Lactobacillus casei Zhang significantly increased numbers of Lactobacillus and Bifidobacterium and decreased Clostridium in the intestine (p < 0.01). Meanwhile, liver glycogen contents were significantly decreased (p < 0.05). In preventive group, accompanied by significantly lower insulin and GLP-2 levels (p < 0.05), L. casei Zhang prevented rats from an increase in oral glucose tolerance area under curve (AUC) which was significant in hyperinsulinemia group (p < 0.05). In therapeutic group, L. casei Zhang administration possessed improved glucose tolerance (p < 0.05), which were associated with increased osteocalcin level (p < 0.01), improved intestinal bile acids secretion (p = 0.060), decreased serum MDA levels (p < 0.05) and upregulation of LXR-α, PPAR-γ and AdipoR2 gene expression, as well as an increase in Bacteroides fragilis (p < 0.05).
Conclusions
Lactobacillus casei Zhang administration exert both preventive and ameliorative effect on oral glucose tolerance AUC in IGT rats but may be via different mechanisms. L. casei Zhang could prevent rats from increased AUC through GLP-2 lowering, while the ameliorative effect in high-fructose-fed post-adolescent rats may be via B. fragilis enriched vitamin K2-dependent osteocalcin mechanism in which AdipoR2, LXR-α and PPAR-γ signaling were involved.








Similar content being viewed by others
Abbreviations
- AdipoR2:
-
Adiponectin receptor 2
- AUC:
-
Area under curve
- GLP-2:
-
Glucagon-like peptide-2
- HFS:
-
High fructose syrup
- IGT:
-
Impaired glucose tolerance
- IR:
-
Insulin resistance
- LXR-α:
-
Liver X receptor-α
- MDA:
-
Malondialdehyde
- OGTT:
-
Oral glucose tolerance test
- PPAR-γ:
-
Peroxisome proliferator-activated receptor gamma
- SD:
-
Sprague Dawley
- VKORC1:
-
Vitamin K epoxide reductase complex subunit 1
References
Bray GA, Nielsen SJ, Popkin BM (2004) Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr 79:537–543
Moeller SM (2009) The effects of high fructose syrup. J Am Coll Nutr 28:619–626
Johnson RJ, Segal MS, Sautin Y, Nakagawa T, Feig DI, Kang DH, Gersch MS, Benner S, Sánchez-Lozada LG (2007) Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am J Clin Nutr 86:899–906
Stanhope KL, Schwarz JM, Keim NL et al (2009) Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest 119:1322–1334
Reaven GM (1995) Pathophysiology of insulin resistance in human disease. Physiol Rev 75:473–486
Yadav H, Jain S, Sinha PR (2007) Antidiabetic effect of probiotic dahi containing Lactobacillus acidophilus and Lactobacillus casei in high fructose fed rats. Nutrition 23:62–68
Yadav H, Jain S, Sinha PR (2008) Oral administration of dahi containing probiotic Lactobacillus acidophilus and Lactobacillus casei delayed the progression of streptozotocin-induced diabetes in rats. J Dairy Res 75:189–195
Yadav H, Jain S, Sinha PR (2008) The effect of probiotic dahi containing Lactobacillus acidophilus and Lactobacillus casei on gastropathic consequences in diabetic rats. J Med Food 11:62–68
Tabuchi M, Ozaki M, Tamura A, Yamada N, Ishida T, Hosoda M, Hosono A (2003) Antidiabetic effect of Lactobacillus GG in streptozotocin-induced diabetic rats. Biosci Biotechnol Biochem 67:1421–1424
Naito E, Yoshida Y, Makino K, Kounoshi Y, Kunihiro S, Takahashi R, Matsuzaki T, Miyazaki K, Ishikawa F (2011) Beneficial effect of oral administration of Lactobacillus casei strain Shirota on insulin resistance in diet-induced obesity mice. J Appl Microbiol 110:650–657
Yun SI, Park HO, Kang JH (2009) Effect of Lactobacillus gasseri BNR17 on blood glucose levels and body weight in a mouse model of type 2 diabetes. J Appl Microbiol 107:1681–1686
Andersson U, Bränning C, Ahrné S, Molin G, Alenfall J, Önning G, Nyman M, Holm C (2010) Probiotics lower plasma glucose in the high-fat fed C57BL/6 J mouse. Benef Microbes 1:189–196
Ya T, Zhang Q, Chu F, Merritt J, Bilige M, Sun T, Du R, Zhang H (2008) Immunological evaluation of Lactobacillus casei Zhang: a newly isolated strain fromkoumiss in Inner Mongolia, China. BMC Immunol 19(9):68
Zhong Z, Zhang W, Du R, Meng H, Zhang H (2012) Effect of Lactobacillus casei Zhang on global gene expression in the liver of hypercholesterolemic rats. Eur J Lipid Sci Technol 114:244–252
McGuinness Owen P, Alan D (2003) Effects of fructose on hepatic glucose metabolism. Curr Opin Clin Nutr Metab Care 6:441–448
Greaves P (2007) Histopathology of preclinical toxicity studies, Third edition: interpretation and relevance in drug safety evaluation. Elsevier, USA, pp 533–534
Gil L, Siems W, Mazurek B, Gross J, Schroeder P, Voss P, Grune T (2006) Age-associated analysis of oxidative stress parameters in human plasma and erythrocytes. Free Radic Res 40:495–505
Zhang Y, Du R, Wang L, Zhang H (2010) The antioxidative effects of probiotic Lactobacillus casei Zhang on the hyperlipidemic rats. Eur Food Res Technol 231:151–158
Neyrinck AM, Possemiers S, Druart C, Van de Wiele T, De Backer F, Cani PD, Larondelle Y, Delzenne NM (2011) Prebiotic effects of wheat arabinoxylan related to the increase in bifidobacteria, roseburia and Bacteroides/Prevotella in diet-induced obese mice. PLoS ONE 6:e20944
Xiao Q, Boushey RP, Drucker DJ, Brubaker PL (1999) Secretion of the intestinotropic hormone glucagon-like peptide 2 is differentially regulated by nutrients in humans. Gastroenterology 117:99–105
Haidari M, Leung N, Mahbub F, Uffelman KD, Kohen-Avramoglu R, Lewis GF, Adeli K (2002) Fasting and postprandial overproduction of intestinally derived lipoproteins in an animal model of insulin resistance. Evidence that chronic fructose feeding in the hamster is accompanied by enhanced intestinal de novo lipogenesis and ApoB48-containing lipoprotein overproduction. J Biol Chem 277:31646–31655
Hsieh J, Longuet C, Maida A, Bahrami J, Xu E, Baker CL, Brubaker PL, Drucker DJ, Adeli K (2009) Glucagon-like peptide-2 increases intestinal lipid absorption and chylomicron production via CD36. Gastroenterology 137:997–1005
Federico LM, Naples M, Taylor D et al (2006) Intestinal insulin resistance and aberrant production of apolipoprotein B48 lipoproteins in an animal model of insulin resistance and metabolic dyslipidemia: evidence for activation of protein tyrosine phosphatase-1B, extracellular signal-related kinase, and sterol regulatory elementbinding protein-1c in the fructose-fed hamster intestine. Diabetes 55:1316–1326
Larsen N, Vogensen FK, van den Berg FW et al (2010) Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE 5:e9085
Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, Zhang Z, Kim JK, Mauvais-Jarvis F, Ducy P, Karsenty G (2007) Endocrine regulation of energy metabolism by the skeleton. Cell 130:456–469
Fukumoto S, Martin TJ (2009) Bone as an endocrine organ. Trends Endocrinol Metab 20:230–236
Naughton V, McSorley E, Naughton PJ (2011) Changes in calcium status in aged rats fed Lactobacillus GG and Bifidobacterium lactis and oligofructose-enriched inulin. Appl Physiol Nutr Metab 36:161–165
Choi HJ, Yu J, Choi H, An JH, Kim SW, Park KS, Jang HC, Kim SY, Shin CS (2011) Vitamin K2 supplementation improves insulin sensitivity via osteocalcin metabolism: a placebo-controlled trial. Diabetes Care 34:e147
Mathers JC, Fernandez F, Hill MJ, McCarthy PT, Shearer MJ, Oxley A (1990) Dietary modification of potential vitamin K supply from enteric bacterial menaquinones in rats. Br J Nutr 63:639–652
Yoshida M, Jacques PF, Meigs JB, Saltzman E, Shea MK, Gundberg C, Dawson-Hughes B, Dallal G, Booth SL (2008) Effect of vitamin K supplementation on insulin resistance in older men and women. Diabetes Care 31:2092–2096
Beulens JW, van der A DL, Grobbee DE, Sluijs I, Spijkerman AM, van der Schouw YT (2010) Dietary phylloquinone and menaquinones intakes and risk of type 2 diabetes. Diabetes Care 33:1699–1705
Olson RE (1984) The function and metabolism of vitamin K. Ann Rev Nutr 4:281–337
Shea MK, Gundberg CM, Meigs JB, Dallal GE, Saltzman E, Yoshida M, Jacques PF, Booth SL (2009) Gamma-carboxylation of osteocalcin and insulin resistance in older men and women. Am J Clin Nutr 90:1230–1235
Yamauchi T, Nio Y, Maki T et al (2007) Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med 13:332–339
López-Bermejo A, Botas-Cervero P, Ortega-Delgado F, Delgado E, García-Gil MM, Funahashi T, Ricart W, Fernández-Real JM (2008) Association of ADIPOR2 with liver function tests in type 2 diabetic subjects. Obesity 16:2308–2313
Nerstedt A, Nilsson EC, Ohlson K, Håkansson J, Thomas Svensson L, Löwenadler B, Svensson UK, Mahlapuu M (2007) Administration of Lactobacillus evokes coordinated changes in the intestinal expression profile of genes regulating energy homeostasis and immune phenotype in mice. Br J Nutr 97:1117–1127
Kadooka Y, Sato M, Imaizumi K, Ogawa A, Ikuyama K, Akai Y, Okano M, Kagoshima M, Tsuchida T (2010) Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur J Clin Nutr 64:636–643
Laffitte BA, Chao LC, Li J, Walczak R, Hummasti S, Joseph SB, Castrillo A, Wilpitz DC, Mangelsdorf DJ, Collins JL, Saez E, Tontonoz P (2003) Activation of liver X receptor improves glucose tolerance through coordinate regulation of glucose metabolism in liver and adipose tissue. Proc Natl Acad Sci USA 100:5419–5424
Huang Y, Zheng Y (2010) The probiotic Lactobacillus acidophilus reduces cholesterol absorption through the down-regulation of Niemann-Pick C1-like 1 in Caco-2 cells. Br J Nutr 103:473–478
Zhang Y, Du R, He Q, Li H, Zhang H (2012) Beneficial effects of Lactobacillus casei Zhang on liver lipids of diet-induced hypercholesterolemia rats. Chin Agric Sci (in Chinese). doi:10.3864/j.issn.0578-1752.2012.05.015
Takebayashi K, Aso Y, Inukai T (2010) Role of bile acid sequestrants in the treatment of type 2 diabetes. World J Diabetes 1:146–152
Olefsky JM, Saltiel AR (2000) PPAR gamma and the treatment of insulin resistance. Trends Endocrinol Metab 11:362–368
Hong C, Tontonoz P (2008) Coordination of inflammation and metabolism by PPAR and LXR nuclear receptors. Curr Opin Genet Dev 18:461–467
Kelly D, Campbell JI, King TP, Grant G, Jansson EA, Coutts AG et al (2004) Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-gamma and RelA. Nat Immunol 5:104–112
Zhao X, Higashikawa F, Noda M, Kawamura Y, Matoba Y, Kumagai T, Sugiyama M (2012) The obesity and fatty liver are reduced by plant-derived pediococcus pentosaceus LP28 in high fat diet-induced obese mice. PLoS One 7:e30696
Acknowledgments
We thank Dr. Dongmin Liu from Fralin Life Science Institute of Virgina Tech for revising this article. This research was supported by National Natural Science Foundation of China (Grant No. 31025019), the Innovation Team Development of the Ministry of Education of China (Grant No. IRT0967), National Basic Research Program of China (973 Program) (2012CB720802), Hi-Tech Research and Development Program of China (863 Planning, Grant No. 2011AA100901, 2011AA100902) and the Earmarked Fund for Modern Agro-industry Technology Research System (Grant No. nycytx-0501).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, Y., Wang, L., Zhang, J. et al. Probiotic Lactobacillus casei Zhang ameliorates high-fructose-induced impaired glucose tolerance in hyperinsulinemia rats. Eur J Nutr 53, 221–232 (2014). https://doi.org/10.1007/s00394-013-0519-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-013-0519-5