Abstract
Purpose
Hypolipidemic and/or hypocholesterolemic effects are presumed for dietary milk phospholipid (PL) as well as plant sterol (PSt) supplementation. The aim was to induce changes in plasma lipid profile by giving different doses of milk PL and a combination of milk PL with PSt to healthy volunteers.
Methods
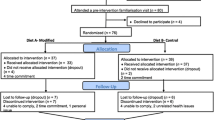
In an open-label intervention study, 14 women received dairy products enriched with moderate (3 g PL/day) or high (6 g PL/day) dose of milk PL or a high dose of milk PL combined with PSt (6 g PL/day + 2 g PSt/day) during 3 periods each lasting 10 days.
Results
Total cholesterol concentration and HDL cholesterol concentration were reduced following supplementation with 3 g PL/day. No significant change in LDL cholesterol concentration was found compared with baseline. High PL dose resulted in an increase of LDL cholesterol and unchanged HDL cholesterol compared with moderate PL dose. The LDL/HDL ratio and triglyceride concentration remained constant within the study. Except for increased phosphatidyl ethanolamine concentrations, plasma PL concentrations were not altered during exclusive PL supplementations. A combined high-dose PL and PSt supplementation led to decreased plasma LDL cholesterol concentration, decreased PL excretion, increased plasma sphingomyelin/phosphatidyl choline ratio, and significant changes in plasma fatty acid distribution compared with exclusive high-dose PL supplementation.
Conclusion
Milk PL supplementations influence plasma cholesterol concentrations, but without changes of LDL/HDL ratio. A combined high-dose milk PL and PSt supplementation decreases plasma LDL cholesterol concentration, but it probably enforces absorption of fatty acids or fatty acid-containing hydrolysis products that originated during lipid digestion.


Similar content being viewed by others
Abbreviations
- CD36:
-
Cluster of differentiation 36
- DM:
-
Dry matter
- FA:
-
Fatty acid
- FABP:
-
Fatty acid binding protein
- FAME:
-
Fatty acid methyl ester
- MUFA:
-
Mono-unsaturated fatty acid
- PC:
-
Phosphatidyl choline
- PE:
-
Phosphatidyl ethanolamine
- PI:
-
Phosphatidyl inositol
- PL:
-
Phospholipid
- PSt:
-
Plant sterol
- PUFA:
-
Poly-unsaturated fatty acid
- SFA:
-
Saturated fatty acid
- SM:
-
Sphingomyelin
- SMFA:
-
Milk sphingomyelin-related fatty acid
- SR-BI:
-
Scavenger receptor class B type I
- TG:
-
Triglyceride
References
Bitman J, Wood DL (1990) Changes in milk fat phospholipids during lactation. J Dairy Sci 73:1208–1216
Nyberg L, Duan RD, Nilsson A (1998) Sphingomyelin: a dietary component with structural and biological function. Prog Colloid Polym Sci 108:119–128
Cohn JS, Kamili A, Wat E, Chung RWS, Tandy S (2010) Reduction in intestinal cholesterol absorption by various food components: mechanisms and implications. Atheroscler Suppl 11:45–48
Tso P, Fujimoto K (1991) The absorption and transport of lipids by the small intestine. Brain Res Bull 27:477–482
Ikeda I, Imaizumi K, Sugano M (1987) Absorption and transport of base moieties of phosphatidylcholine and phosphatidylethanolamine in rats. Biochim Biophys Acta 921:245–253
Richmond BL, Boileau AC, Zheng S, Huggins KW, Granholm NA, Tso P, Hui DY (2001) Compensatory phospholipids digestion is required for cholesterol absorption in pancreatic phospholipase A2-deficient mice. Gastroenterology 120:1193–1202
Nilsson A (1968) Metabolism of sphingomyelin in the intestinal tract of the rat. Biochim Biophys Acta 164:575–584
Schmelz EM, Crall KJ, Larocque R, Dillehay DL, Merrill AH Jr (1994) Uptake and metabolism of sphingolipids in isolated intestinal loops of mice. J Nutr 124:702–712
Iqbal J, Hussain MM (2009) Intestinal lipid absorption. Am J Physiol Endocrinol Metab 296:E1183–E1194
Kamili A, Wat E, Chung RWS, Tandy S, Weir JM, Meikle PJ, Cohn JS (2010) Hepatic accumulation of intestinal cholesterol is decreased and fecal cholesterol excretion is increased in mice fed a high-fat diet supplemented with milk phospholipids. Nutr Metab 7:90
Wat E, Tandy S, Kapera E, Kamili A, Chung RWS, Brown A, Rowney M, Cohn JS (2009) Dietary phospholipids-rich dairy milk extract reduces hepatomegaly, hepatic steatosis and hyperlipidemia in mice fed a high-fat diet. Atherosclerosis 205:144–150
Müller H, Hellgren LI, Olsen E, Skrede A (2004) Lipids rich in phosphatidylethanolamine from natural gas-utilizing bacteria reduce plasma cholesterol and classes of phospholipids: a comparison with soybean oil. Lipids 39:833–841
Jiang Y, Noh SK, Koo SI (2001) Egg phosphatidylcholine decreases the lymphatic absorption of cholesterol in rats. J Nutr 131:2358–2363
Nieuwenhuizen WF, Duivenvoorden I, Voshol PJ, Rensen PCN, van Duyvenvoorde W, Romijn JA, Emeis JJ, Havekes LM (2007) Dietary sphingolipids lower plasma cholesterol and triacylglycerol and prevent liver steatosis. Eur J Lipid Sci Technol 109:994–997
Nyberg L, Duan RD, Nilsson A (2000) A mutual inhibitory effect on absorption of sphingomyelin and cholesterol. J Nutr Biochem 11:244–249
Micallef MA, Garg ML (2009) Beyond blood lipids: phytosterols, statins and omega-3 polyunsaturated fatty acid therapy for hyperlipidemia. J Nutr Biochem 20:927–939
Rideout TC, Harding SV, Mackay D, Abumweis SS, Jones PJH (2010) High basal fractional cholesterol synthesis is associated with nonresponse of plasma LDL cholesterol to plant sterol therapy. Am J Clin Nutr 92:41–46
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Xu G, Waki H, Kon K, Ando S (1996) Thin-layer chromatography of phospholipids and their lyso forms: application to determination of extracts from rat hippocampal CA1 region. Microchem J 53:29–33
Colarow L (1990) Quantitative transmittance densitometry of phospholipids after their specific detection with a molybdate reagent on silica gel plates. J Planar Chromatogr Mod TLC 3:228–231
Lendrath G, Bonekamp-Nasner A, Kraus LJ (1991) Analytical possibilities of qualitative and quantitative determination of phospholipids of different sources. Eur J Lipid Sci Tech 93:53–61
Rouser G, Fleischer S, Yamamoto A (1970) Two dimensional thin layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids 5:494–496
Goodman DS, Shiratori T (1964) Fatty acid composition of human plasma lipoprotein fractions. J Lipid Res 5:307–313
McDowell AKR (1958) Phospholipids in New Zealand dairy products: II. Seasonal variations in the phospholipid content of butter and of milk and cream. J Dairy Res 25:202–214
Singleton JA, Pattee HE (1981) Computation of conversion factors to determine the phospholipid content in peanut oils. J Am Oil Chem Soc 58:873–875
Valeur A, Olsson NU, Kaufmann P, Wada S, Kroon CG, Westerdahl G, Odham G (1994) Quantification and comparison of some natural sphingomyelins by on-line high-performance liquid chromatography/discharge-assisted thermospray mass spectrometry. Biol Mass Spectrom 23:313–319
Keller S, Jahreis G (2004) Determination of underivatised sterols and bile acid trimethyl silyl ether methyl esters by gas chromatography-mass spectrometry-single ion monitoring in faeces. J Chromatogr B Biomed Sci Appl 813:199–207
D-A-CH (2008) The reference values for nutrient intake. Umschau/Braus, Frankfurt/Main
Ohlsson L, Burling H, Nilsson A (2009) Long term effect on human plasma lipoproteins of a formulation enriched in butter milk polar lipid. Lipids Health Dis. doi:10.1186/1476-511X-8-44
Ohlsson L (2010) Dairy products and plasma cholesterol levels. Food Nutr Res. doi:10.3402/fnr.v54i0.5124
Tholstrup T, Hoy CE, Andersen LN, Christensen RDK, Sandström B (2004) Does fat in milk, butter and cheese affect blood lipids and cholesterol differently? J Am Coll Nutr 23:169–176
Andrade S, Borges N (2009) Effect of fermented milk containing Lactobacillus acidophilus and Bifidobacterium longum on plasma lipids of women with normal or moderately elevated cholesterol. J Dairy Res 76:469–474
Ridgway ND (2000) Interactions between metabolism and intracellular distribution of cholesterol and sphingomyelin. Biochim Biophys Acta 1484:129–141
Imaizumi K, Tominaga A, Sato M, Sugano M (1992) Effects of dietary sphingolipids on levels of serum and liver lipids in rats. Nutr Res 12:543–548
Merrill AH, Jones DD (1990) An update of the enzymology and regulation of sphingomyelin metabolism. Biochim Biophys Acta 1044:1–12
Tijburg LBM, Geelen MJH, van Golde LMG (1989) Regulation of the biosynthesis of triacylglycerol, phosphatidylcholine and phosphatidylethanolamine in the liver. Biochim Biophys Acta 1004:1–19
Sundler R, Akesson B (1975) Regulation of phospholipids biosynthesis in isolated rat hepatocytes. Effect of different substrates. J Biol Chem 250:3359–3367
Levy E, Spahis S, Sinnett D, Peretti N, Maupas-Schwalm F, Delvin E, Lambert M, Lavoie MA (2007) Intestinal cholesterol transport proteins: an update and beyond. Curr Opin Lipidol 18:310–318
Gupta AK, Savopoulos CG, Ahuja J, Hatzitolios AI (2011) Role of phytosterols in lipid-lowering: current perspectives. Q J Med 104:301–308
Salen G, Shefer S, Nguyen L, Ness GC, Tint GS, Shore V (1992) Sitosterolemia. J Lipid Res 33:945–955
Vergès B, Athias A, Petit JM, Brindisi MC (2009) Extravascular lipid deposit (xanthelasma) induced by a plant sterol-enriched margarine. BMJ Case Rep. doi:10.1136/bcr.10.2008.1108
Kelly ER, Plat J, Mensink RP, Berendschot TT (2011) Effects of long term plant sterol and stanol consumption on the retinal vasculature: a randomized controlled trial in statin users. Atherosclerosis 214:225–230
Teupser D, Baber R, Ceglarek U et al (2010) Genetic regulation of serum phytosterol levels and risk of coronary artery disease. Circ Cardiovasc Gene 3:331–339
Assmann G, Cullen P, Erbey J, Ramey DR, Kannenberg F, Schulte H (2006) Plasma sitosterol elevations are associated with an increased incidence of coronary events in men: results of a nested-control analysis of the prospective cardiovascular münster (PROCAM) study. Nutr Metab Cardiovas 16:13–21
Vanmierlo T, Weingärtner O, van der Pol S, Husche C, Kerksiek A, Friedrichs S, Sijbrands E, Steinbusch H, Grimm M, Hartmann T, Laufs U, Böhm M, de Vries HE, Mulder M, Lütjohann D (2012) Dietary intake of plant sterols stably increases plant sterol levels in the murine brain. J Lipid Res 53:726–735
Weingärtner O, Lütjohann D, Ji S, Weisshoff N, List F, Sudhop T, von Bergmann K, Gertz K, König J, Schäfers HJ, Endres M, Böhm M, Laufs U (2008) Vascular effects of diet supplementation with plant sterols. J Am Coll Cardiol 51:1553–1561
Kreuzer J (2011) Phytosterols and phytostanols: is it time to rethink that supplemented margarine? Cardiovasc Res 90:397–398
Jiang XC, Paultre F, Pearson TA, Reed RG, Francis CK, Lin M, Berglund L, Tall AR (2000) Plasma sphingomyelin level as a risk factor for coronary artery disease. Arterioscler Thromb Vasc Biol 20:2614–2618
Nilsson A, Duan RD (2006) Absorption and lipoprotein transport of sphingomyelin. J Lipid Res 47:154–171
Schlitt A, Hojjati MR, von Gizycki H, Lackner KJ, Blankenberg S, Schwaab B, Meyer J, Rupprecht HJ, Jiang XC (2005) Serum sphingomyelin levels are related to the clearance of postprandial remnant-like particles. J Lipid Res 46:196–200
King IB, Lemaitre RN, Kestin M (2006) Effect of a low-fat diet on fatty acid composition in red cells, plasma phospholipids, and cholesterol esters: investigation of a biomarker of total fat intake. Am J Clin Nutr 83:227–236
Hodson L, Skeaff CM, Fielding BA (2008) Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res 47:348–380
Brufau G, Canela MA, Rafecas M (2006) A high-saturated fat diet enriched with phytosterol and pectin affects the fatty acid profile in guinea pigs. Lipids 41:159–168
Brufau G, Canela MA, Rafecas M (2007) Phytosterols, but not pectin, added to a high-saturated-fat diet modify saturated fatty acid excretion in relation to chain length. J Nutr Biochem 18:580–586
Wang DQH, Carey MC (1996) Complete mapping of crystallization pathways during cholesterol precipitation from model bile: influence of physical-chemical variables of pathophysiologic relevance and identification of a stable liquid crystalline state in cold, dilute and hydrophilic bile salt-containing systems. J Lipid Res 37:606–630
Bietrix F, Yan D, Nauze M, Rolland C, Bertrand-Michel J, Comera C, Schaak S, Barbaras R, Groen AK, Perret B, Terce F, Collet X (2006) Accelerated lipid absorption in mice overexpressing intestinal SR-BI. J Biol Chem 281:7214–7219
Engelmann B, Wiedmann MK (2010) Cellular phospholipid uptake: flexible paths to coregulate the functions of intracellular lipids. Biochim Biophys Acta 1801:609–616
Poirier H, Degrace P, Niot I, Bernard A, Besnard P (1996) Localization and regulation of the putative membrane fatty-acid transporter (FAT) in the small intestine. Comparison with fatty acid-binding proteins (FABP). Eur J Biochem 238:368–373
Nauli AM, Nassir F, Zheng S, Yang Q, Lo CM, Vonlehmden SB, Lee D, Jandacek RJ, Abumrad NA, Tso P (2006) CD36 is important for chylomicron formation and secretion and may mediate cholesterol uptake in the proximal intestine. Gastroenterology 131:1197–1207
Stremmel W (1988) Uptake of fatty acids by jejunal mucosal cells is mediated by a fatty acid binding membrane protein. J Clin Invest 82:2001–2010
Méndez-González J, Süren-Castillo S, Calpe-Berdiel L, Rotllan N, Vázquez-Carrera M, Escolà-Gil JC, Blanco-Vaca F (2010) Disodium ascorbyl phytostanol phosphate (FM-VP4), a modified phytostanol, is a highly active hypocholesterolaemic agent that affects the enterohepatic circulation of both cholesterol and bile acids in mice. Br J Nutr 103:153–160
Ruiu G, Pinach S, Veglia F, Gambino R, Marena S, Uberti B, Alemanno N, Burt D, Pagano G, Cassader M (2009) Phytosterol-enriched yogurt increases LDL affinity and reduces CD36 expression in polygenic hypercholesterolemia. Lipids 44:153–160
Acknowledgments
We would like to thank our volunteers for their engaged participation in the study. Nasim Kroegel is acknowledged for language editing. This research project was supported by the German Ministry of Economics and Technology (via AiF) and the FEI (Forschungskreis der Ernährungsindustrie e.V., Bonn). Project AiF 316ZBG.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Keller, S., Malarski, A., Reuther, C. et al. Milk phospholipid and plant sterol-dependent modulation of plasma lipids in healthy volunteers. Eur J Nutr 52, 1169–1179 (2013). https://doi.org/10.1007/s00394-012-0427-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-012-0427-0