Abstract
Purpose
Oxidative stress and inflammation contribute to hepatic injury after hemorrhage/resuscitation (H/R). Natural plant polyphenols, i.e., green tea extract (GTE) possess high anti-oxidant and anti-inflammatory activities in various models of acute inflammation. However, possible protective effects and feasible mechanisms by which plant polyphenols modulate pro-inflammatory, apoptotic, and oxidant signaling after H/R in the liver remain unknown. Therefore, we investigated the effects of GTE and its impact on the activation of NF-kappaB in the pathogenesis of hepatic injury induced by H/R.
Methods
Twenty-four female LEWIS rats (180–250 g) were fed a standard chow (ctrl) or a diet containing 0.1% polyphenolic extracts (GTE) from Camellia sinensis starting 5 days before H/R. Rats were hemorrhaged to a mean arterial pressure of 30 ± 2 mmHg for 60 min and resuscitated (H/R and GTE H/R groups). Control groups (sham, ctrl, and GTE) underwent surgical procedures without H/R. Two hours after resuscitation, tissues were harvested.
Results
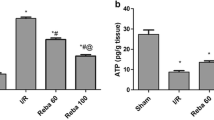
Plasma alanine aminotransferase (ALT) and lactate dehydrogenase (LDH) increased 3.5-fold and fourfold, respectively, in vehicle-treated rats as compared to GTE-fed rats. Histopathological analysis revealed significantly decreased hepatic necrosis and apoptosis in GTE-fed rats after H/R. Real-time PCR showed that GTE diminished gene expression of pro-apoptotic caspase-8 and Bax, while anti-apoptotic Bcl-2 was increased after H/R. Hepatic oxidative (4-hydroxynonenal) and nitrosative (3-nitrotyrosine) stress as well as systemic IL-6 level and hepatic IL-6 mRNA were markedly reduced in GTE-fed rats compared with controls after H/R. Plant polyphenols also decreased the activation of both JNK and NFκB.
Conclusions
Taken together, GTE application blunts hepatic damage, apoptotic, oxidative, and pro-inflammatory changes after H/R. These results underline the important roles of JNK and NF-kappaB in inflammatory processes after H/R and the beneficial impact of plant polyphenols in preventing their activation.






Similar content being viewed by others
References
Peden M, Hyder A (2002) Road traffic injuries are a global public health problem. BMJ 324(7346):1153
Mock C, Joshipura M, Goosen J, Maier R (2006) Overview of the essential trauma care project. World J Surg 30(6):919–929
Baue AE, Durham R, Faist E (1998) Systemic inflammatory response syndrome (SIRS), multiple organ dysfunction syndrome (MODS), multiple organ failure (MOF): are we winning the battle? Shock 10(2):79–89
Bogner V, Keil L, Kanz KG, Kirchhoff C, Leidel BA, Mutschler W, Biberthaler P (2009) Very early posttraumatic serum alterations are significantly associated to initial massive RBC substitution, injury severity, multiple organ failure and adverse clinical outcome in multiple injured patients. Eur J Med Res 14(7):284–291
Moore FA, Sauaia A, Moore EE, Haenel JB, Burch JM, Lezotte DC (1996) Postinjury multiple organ failure: a bimodal phenomenon. J Trauma 40(4):501–510
Hietbrink F, Koenderman L, Rijkers G, Leenen L (2006) Trauma: the role of the innate immune system. World J Emerg Surg 1:15
Partrick DA, Moore FA, Moore EE, Barnett CC Jr, Silliman CC (1996) Neutrophil priming and activation in the pathogenesis of postinjury multiple organ failure. New Horiz 4(2):194–210
Botha AJ, Moore FA, Moore EE, Kim FJ, Banerjee A, Peterson VM (1995) Postinjury neutrophil priming and activation: an early vulnerable window. Surgery 118(2):358–364
Redl H, Gasser H, Schlag G, Marzi I (1993) Involvement of oxygen radicals in shock related cell injury. Br Med Bull 49(3):556–565
Passos JF, Saretzki G, von Zglinicki ZT (2007) DNA damage in telomeres and mitochondria during cellular senescence: is there a connection? Nucleic Acids Res 35(22):7505–7513
Akgur FM, Brown MF, Zibari GB, McDonald JC, Epstein CJ, Ross CR, Granger DN (2000) Role of superoxide in hemorrhagic shock-induced P-selectin expression. Am J Physiol Heart Circ Physiol 279(2):H791–H797
Relja B, Schwestka B, Lee VS, Henrich D, Czerny C, Borsello T, Marzi I, Lehnert M (2009) Inhibition of c-Jun N-terminal kinase after hemorrhage but before resuscitation mitigates hepatic damage and inflammatory response in male rats. Shock 32(5):509–516
Meng ZH, Dyer K, Billiar TR, Tweardy DJ (2001) Essential role for IL-6 in postresuscitation inflammation in hemorrhagic shock. Am J Physiol Cell Physiol 280(2):C343–C351
Lluis JM, Llacuna L, von Montfort MC, Barcena C, Enrich C, Morales A, Fernandez-Checa JC (2009) GD3 synthase overexpression sensitizes hepatocarcinoma cells to hypoxia and reduces tumor growth by suppressing the cSrc/NF-kappaB survival pathway. PLoS One 4(11):e8059
Lee CW, Lin CC, Lin WN, Liang KC, Luo SF, Wu CB, Wang SW, Yang CM (2007) TNF-alpha induces MMP-9 expression via activation of Src/EGFR, PDGFR/PI3K/Akt cascade and promotion of NF-kappaB/p300 binding in human tracheal smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 292(3):L799–L812
Guha M, Mackman N (2001) (2001) LPS induction of gene expression in human monocytes. Cell Signal 13(2):85–94
Hierholzer C, Harbrecht B, Menezes JM, Kane J, MacMicking J, Nathan CF, Peitzman AB, Billiar TR, Tweardy DJ (1998) Essential role of induced nitric oxide in the initiation of the inflammatory response after hemorrhagic shock. J Exp Med 187(6):917–928
Zingarelli B, Sheehan M, Wong HR (2003) Nuclear factor-kappaB as a therapeutic target in critical care medicine. Crit Care Med 31(1):S105–S111
Gaddipati JP, Sundar SV, Calemine J, Seth P, Sidhu GS, Maheshwari RK (2003) Differential regulation of cytokines and transcription factors in liver by curcumin following hemorrhage/resuscitation. Shock 19(2):150–156
Baeuerle PA, Baltimore D (1998) A 65-kappaD subunit of active NF-kappaB is required for inhibition of NF-kappaB by I kappaB. Genes Dev 3(11):1689–1698
Hara Y (1994) Antioxidative action of tea polyphenols: Part 1. Am Biotechnol Lab 12(8):48
Zhao BL, Li XJ, He RG, Cheng SJ, Xin WJ (1989) Scavenging effect of extracts of green tea and natural antioxidants on active oxygen radicals. Cell Biophys 14(2):175–185
Kuriyama S (2008) The relation between green tea consumption and cardiovascular disease as evidenced by epidemiological studies. J Nutr 138(8):1548S–1553S
Kuriyama S (2010) Green tea consumption and prevention of coronary artery disease. Circ J 74(2):248–249
Zhong Z, Froh M, Connor HD, Li X, Conzelmann LO, Mason RP, Lemasters JJ, Thurman RG (2002) Prevention of hepatic ischemia-reperfusion injury by green tea extract. Am J Physiol Gastrointest Liver Physiol 283(4):G957–G964
Zhong Z, Connor HD, Froh M, Lind H, Bunzendahl H, Mason RP, Thurman RG, Lemasters JJ (2004) Polyphenols from Camellia sinenesis prevent primary graft failure after transplantation of ethanol-induced fatty livers from rats. Free Radic Biol Med 36(10):1248–1258
Fiorini RN, Donovan JL, Rodwell D, Evans Z, Cheng G, May HD, Milliken CE, Markowitz JS, Campbell C, Haines JK, Schmidt MG, Chavin KD (2005) Short-term administration of (-)-epigallocatechin gallate reduces hepatic steatosis and protects against warm hepatic ischemia/reperfusion injury in steatotic mice. Liver Transpl 11(3):298–308
Relja B, Lehnert M, Seyboth K, Bormann F, Hohn C, Czerny C, Henrich D, Marzi I (2010) Simvastatin reduces mortality and hepatic injury after hemorrhage/resuscitation in rats. Shock 34(1):46–54
Lehnert M, Arteel GE, Smutney OM, Conzelmann LO, Zhong Z, Thurman RG, Lemasters JJ (2003) Dependence of liver injury after hemorrhage/resuscitation in mice on NADPH oxidase-derived superoxide. Shock 19(4):345–351
Lehnert M, Relja B, Sun-Young L, V, Schwestka B, Henrich D, Czerny C, Froh M, Borsello T, Marzi I (2008) A peptide inhibitor of C-jun N-terminal kinase modulates hepatic damage and the inflammatory response after hemorrhagic shock and resuscitation. Shock 30(2):159–165
Jaeschke H, Mitchell JR (1989) Mitochondria and xanthine oxidase both generate reactive oxygen species in isolated perfused rat liver after hypoxic injury. Biochem Biophys Res Commun 160(1):140–147
Granger DN, Benoit JN, Suzuki M, Grisham MB (1989) Leukocyte adherence to venular endothelium during ischemia-reperfusion. Am J Physiol 257(5 Pt 1):G683–G688
Jaeschke H, Bautista AP, Spolarics Z, Spitzer JJ (1992) Superoxide generation by neutrophils and Kupffer cells during in vivo reperfusion after hepatic ischemia in rats. J Leukoc Biol 52(4):377–382
Farber JL (1994) Mechanisms of cell injury by activated oxygen species. Environ Health Perspect 102(10):17–24
Gujral JS, Bucci TJ, Farhood A, Jaeschke H (2001) Mechanism of cell death during warm hepatic ischemia-reperfusion in rats: apoptosis or necrosis? Hepatology 33(2):397–405
Bendinelli P, Piccoletti R, Maroni P, Bernelli-Zazzera A (1996) The MAP kinase cascades are activated during post-ischemic liver reperfusion. FEBS Lett 398(2–3):193–197
Chan ED, Winston BW, Jarpe MB, Wynes MW, Riches DW (1997) Preferential activation of the p46 isoform of JNK/SAPK in mouse macrophages by TNF alpha. Proc Natl Acad Sci U S A 94(24):13169–13174
McCloskey CA, Kameneva MV, Uryash A, Gallo DJ, Billiar TR (2004) Tissue hypoxia activates JNK in the liver during hemorrhagic shock. Shock 22(4):380–386
Westwick JK, Weitzel C, Leffert HL, Brenner DA (1995) Activation of Jun kinase is an early event in hepatic regeneration. J Clin Invest 95(2):803–810
Lehnert M, Uehara T, Bradford BU, Lind H, Zhong Z, Brenner DA, Marzi I, Lemasters JJ (2006) Lipopolysaccharide-binding protein modulates hepatic damage and the inflammatory response after hemorrhagic shock and resuscitation. Am J Physiol Gastrointest Liver Physiol 291(3):G456–G463
Weston CR, Davis RJ (2007) The JNK signal transduction pathway. Curr Opin Cell Biol 19(2):142–149
Minet E, Michel G, Mottet D, Piret JP, Barbieux A, Raes M, Michiels C (2001) c-JUN gene induction and AP-1 activity is regulated by a JNK-dependent pathway in hypoxic HepG2 cells. Exp Cell Res 265(1):114–124
Schreck R, Rieber P, Baeuerle PA (1991) Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J 10(8):2247–2258
Dudek EJ, Shang F, Taylor A (2001) H(2)O(2)-mediated oxidative stress activates NF-kappa B in lens epithelial cells. Free Radic Biol Med 31(5):651–658
Han YJ, Kwon YG, Chung HT, Lee SK, Simmons RL, Billiar TR, Kim YM (2001) Antioxidant enzymes suppress nitric oxide production through the inhibition of NF-kappa B activation: role of H(2)O(2) and nitric oxide in inducible nitric oxide synthase expression in macrophages. Nitric Oxide 5(5):504–513
Hayden MS, Ghosh S (2004) Signaling to NF-kappaB. Genes Dev 18(18):2195–2224
Kubulus D, Mathes A, Reus E, Pradarutti S, Pavlidis D, Thierbach JT, Heiser J, Wolf B, Bauer I, Rensing H (2009) Endothelin-1 contributes to hemoglobin glutamer-200-mediated hepatocellular dysfunction after hemorrhagic shock. Shock 32(2):179–189
Vallabhaneni R, Kaczorowski DJ, Yaakovian MD, Rao J, Zuckerbraun BS (2010) Heme oxygenase-1 protects against hepatic hypoxia and injury from hemorrhage via regulation of cellular respiration. Shock 33(3):274–281
Meldrum DR, Shenkar R, Sheridan BC, Cain BS, Abraham E, Harken AH (1997) Hemorrhage activates myocardial NFkappaB and increases TNF-alpha in the heart. J Mol Cell Cardiol 29(10):2849–2854
Ayuste EC, Chen H, Koustova E, Rhee P, Ahuja N, Chen Z, Valeri CR, Spaniolas K, Mehrani T, Alam HB (2006) Hepatic and pulmonary apoptosis after hemorrhagic shock in swine can be reduced through modifications of conventional Ringer’s solution. J Trauma 60(1):52–63
Sundar SV, Li YY, Rollwagen FM, Maheshwari RK (2005) Hemorrhagic shock induces differential gene expression and apoptosis in mouse liver. Biochem Biophys Res Commun 332(3):688–696
Yang R, Martin-Hawver L, Woodall C, Thomas A, Qureshi N, Morrison D, Van WC, III (2007) Administration of glutamine after hemorrhagic shock restores cellular energy, reduces cell apoptosis and damage, and increases survival. JPEN J Parenter Enteral Nutr 31(2):94–100
Maitra SR, Bhaduri S, El-Maghrabi MR, Shapiro MJ (2005) Inhibition of matrix metalloproteinase on hepatic transforming growth factor beta1 and caspase-3 activation in hemorrhage. Acad Emerg Med 12(9):797–803
Hurt RT, Zakaria R, Matheson PJ, Cobb ME, Parker JR, Garrison RN (2009) Hemorrhage-induced hepatic injury and hypoperfusion can be prevented by direct peritoneal resuscitation. J Gastrointest Surg 13(4):587–594
Uehara T, Xi P, Bennett B, Satoh Y, Friedman G, Currin R, Brenner DA, Lemasters J (2004) c-Jun N-terminal kinase mediates hepatic injury after rat liver transplantation. Transplantation 78(3):324–332
Guan QH, Pei DS, Liu XM, Wang XT, Xu TL, Zhang GY (2006) Neuroprotection against ischemic brain injury by SP600125 via suppressing the extrinsic and intrinsic pathways of apoptosis. Brain Res 1092(1):36–46
Webster GA, Perkins ND (1999) Transcriptional cross talk between NF-kappaB and p53. Mol Cell Biol 19(5):3485–3495
Barkett M, Gilmore TD (1999) Control of apoptosis by Rel/NF-kappaB transcription factors. Oncogene 18(49):6910–6924
Kim JW, Jin YC, Kim YM, Rhie S, Kim HJ, Seo HG, Lee JH, Ha YL, Chang KC (2009) Daidzein administration in vivo reduces myocardial injury in a rat ischemia/reperfusion model by inhibiting NF-kappaB activation. Life Sci 84(7–8):227–234
Acknowledgments
We thank Kerstin Wilhelm and Minhong Wang for outstanding technical assistance. The study was supported by Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) MA 1119/3-3.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Relja, B., Töttel, E., Breig, L. et al. Plant polyphenols attenuate hepatic injury after hemorrhage/resuscitation by inhibition of apoptosis, oxidative stress, and inflammation via NF-kappaB in rats. Eur J Nutr 51, 311–321 (2012). https://doi.org/10.1007/s00394-011-0216-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-011-0216-1