Abstract
Introduction
TRV027, a ‘biased’ ligand of the angiotensin II type 1 receptor (AT1R), did not affect a composite clinical outcome at 30 days in a phase 2b acute heart failure (AHF) trial (BLAST-AHF).
Methods
Post-hoc analyses from BLAST-AHF (n = 618) examined the effects of TRV027 by baseline systolic blood pressure (SBP) on changes in renal function and 180-day outcomes. Interactions between baseline SBP and select endpoints were identified utilizing a subpopulation treatment effect pattern plots (STEPP) analysis, then grouping of patients by SBP tertile: < 127, ≥ 127 to < 140, and ≥ 140 mmHg.
Results
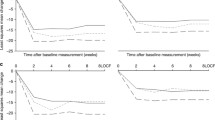
A trend towards increased creatinine in the first 3 days was noted in the lower SBP tertile, while in those in the higher two tertiles, TRV027, especially the 1 mg/h dose, reduced creatinine at days 5 and 30. Beneficial effects on 180-day all-cause mortality and cardiovascular (CV) death or readmission were observed in the two higher SBP tertiles (SBP ≥ 127 mmHg) in the TRV027 1 mg/h dose group (all-cause mortality HR 0.39, 95% CI 0.14–1.06, p = 0.056; CV death or HF/RF rehospitalization HR 0.53, 95% CI 0.28–1.01, p = 0.049), while more adverse outcomes were observed in patients in the lower SBP tertile.
Conclusions
This post-hoc analysis of the BLAST-AHF study suggests contrasting effects of TRV027 by baseline SBP, with trends towards lower 180-day event rates in patients enrolled with higher baseline SBP, especially when given lower doses of TRV027.






Similar content being viewed by others
References
Rajagopal K, Whalen EJ, Violin JD et al (2006) Beta-arrestin2-mediated inotropic effects of the angiotensin II type 1A receptor in isolated cardiac myocytes. Proc Natl Acad Sci USA 103:16284–16289
Soergel DG, Subach RA, Cowan CL, Violin JD, Lark MW (2013) First clinical experience with TRV027: pharmacokinetics and pharmacodynamics in healthy volunteers. J Clin Pharmacol 53:892–899
Felker GM, Butler J, Collins SP, Cotter G, Davison BA, Ezekowitz JA, Filippatos G, Levy PD, Metra M, Ponikowski P, Soergel DG, Teerlink JR, Violin JD, Voors AA, Pang PS (2015) Heart failure therapeutics on the basis of a biased ligand of the angiotensin-2 type 1 receptor. Rationale and design of the BLAST-AHF study (biased ligand of the angiotensin receptor study in acute heart failure). JACC Heart Fail 3:193–201
Heart failure therapeutics on the basis of a biased ligand of the angiotensin-2 type 1 receptor. Rationale and design of the BLAST-AHF study (biased ligand of the angiotensin receptor study in acute heart failure). JACC Heart Fail. 2015; 3: 193–201
Bonetti M, Gelber RD (2004) Patterns of treatment effects in subsets of patients in clinical trials. Biostatistics 5:465–481
Saville BR, Herring AH, Koch GG (2010) A robust method for comparing two treatments in a confirmatory clinical trial via multivariate time-to-event methods that jointly incorporate information from longitudinal and time-to-event data. Stat Med 29:75–85
Sun H, Davison BA, Cotter G, Pencina MJ, Koch GG (2012) Evaluating treatment efficacy by multiple end points in phase II acute heart failure clinical trials: analyzing data using a global method. Circ Heart Fail 5:742–749
Sackner-Bernstein JD1, Skopicki HA, Aaronson KD (2005) Risk of worsening renal function with nesiritide in patients with acutely decompensated heart failure. Circulation 111:1487–1491
Aaronson KD, Sackner-Bernstein J (2006) Risk of death associated with nesiritide in patients with acutely decompensated heart failure. JAMA 296:1465–1466
Voors AA, Davison BA, Felker GM, Ponikowski P, Unemori E, Cotter G et al (2011) Early drop in systolic blood pressure and worsening renal function in acute heart failure: renal results of pre-RELAX-AHF. Eur J Heart Fail 13:961–967
Patel PA, Heizer G, O’Connor CM, Schulte PJ, Dickstein K, Ezekowitz JA et al (2014) Hypotension during hospitalization for acute heart failure is independently associated with 30-day mortality: findings from ASCEND-HF. Circ Heart Fail 7:918–925
Cotter G, Davison B (2014) Intravenous therapies in acute heart failure—lack of effect or lack of well powered studies? Eur J of Heart Fail 16:355–357
Packer M, Colucci W, Fisher L, Massie BM, Teerlink JR, Young J et al (2013) Effect of levosimendan on the short-term clinical course of patients with acutely decompensated heart failure. JACC Heart Fail 1:103–111
Packer M, O’Connor C, McMurray JJ, Wittes J, Abraham WT, Anker SD, Dickstein K, Filippatos G, Holcomb R, Krum H, Maggioni AP, Mebazaa A, Peacock WF, Petrie MC, Ponikowski P, Ruschitzka F, van Veldhuisen DJ, Kowarski LS, Schactman M, Holzmeister J, TRUE-AHF Investigators (2017) Effect of ularitide on cardiovascular mortality in acute heart failure. N Engl J Med 376:1956–1964
Ishihara S, Gayat E, Sato N, Arrigo M, Laribi S, Legrand M, Placido R, Manivet P, Cohen-Solal A, Abraham WT, Jessup M, Mebazaa A (2016) Similar hemodynamic decongestion with vasodilators and inotropes: systematic review, meta-analysis, and meta-regression of 35 studies on acute heart failure. Clin Res Cardiol 105:971–980
Miró Ò, Gil V, Müller C, Mebazaa A, Bueno H, Martín-Sánchez FJ, Herrero P, Jacob J, Llorens P. How does a clinical trial fit into the real world? The RELAX-AHF study population into the EAHFE registry. Clin Res Cardiol 2015; 104: 850 – 60
Kim KS, Abrham D, Williams B, Violin JD, Mao L, Rockman HA (2012) β-Arrestin-biased AT1R stimulation promotes cell survival during acute cardiac injury. Am J Physiol Heart Circ Physiol 303:H1001–H1010
Ivanov M, Mihailović-Stanojević N, Grujić Milanović J, Jovović Đ, Marković-Lipkovski J, Ćirović S, Miloradović Z. Losartan improved antioxidant defense, renal function and structure of postischemic hypertensive kidney. PLoS One 9:e96353
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Drs. Cotter, Davison, and Senger are employees of Momentum Research, which has provided consulting services to NovaCardia, Merck, Corthera, Novartis, Singulex, ChanRx, Laguna Pharmaceuticals, Sorbent Therapeutics, Celyad SA, Trevena, Amgen and Anexon. Butler—Research—NIH, PCORI, and European Union, Consultant to Amgen, Bayer, Boehringer Ingelheim, Cardiocell, Merck, Novartis, Trevena, Relypsa, ZS Pharma. Collins—Research—NIH, PCORI, Novartis, Cardiorentis, Trinity; Consulting: Cardiorentis, Novartis, Trevena, Siemens. Ezekowitz has received grants or honoraria from Novartis, Servier, Bayer, Merck, Trevena, Amgen, Canadian Institutes of Health Research, National Institutes of Health, Heart and Stroke Foundation of Canada. Felker: Consulting for Trevena, Novartis, Amgen, BMS, Stealth Bio-therapeutics, GSK, Medtronic, Myokardia, Grant funding from NIH, AHA, Roche Diagnostics, Otsuka, Novartis, Amgen, Merck. Filippatos: committee fees from Bayer, Novartis, and Servier. Levy- Research- NIH, PCORI, Novartis, Cardiorentis, Trevena; Consulting: Novartis, Cardiorentis, Trevena, ZS Pharma. Metra: Consulting from: Amgen, Bayer, Novartis, Relypsa. Ponikowski: consulting from Bayer, Boehringer Ingelheim, Novartis. Teerlink: Consulting from: Amgen, Bayer, BMS, Cytokinetics, Gilead, Merck, Novartis, Relypsa, Stealth Bio-therapeutics, ZS Pharma; Research funding from: Amgen, Bayer, BMS, Cardio3Biosciences, Medtronic, Novartis, St. Jude, Trevena. Voors: received consultancy fees and/or research grants from: AstraZeneca, Bayer, BMS, Boehringer, Cardio3Biosciences, GSK, Merck/MSD, Novartis, Servier, Sphingotec, Stealth, Trevena, Vifor. Bharucha is an employee of Allergan plc (Forest Labs), a sponsor of this trial. Soergel and Goin are employees of Trevena, the primary sponsor of this trial. Peter S. Pang is or has been in the last one year a Consultant for: BMS, Janssen, Medtronic, the Medicines Company, Novartis, Trevena, scPharmaceuticals, Cardioxyl, Roche Diagnostics, Relypsa, Research Support: Roche, Novartis, PCORI, Indianapolis EMS.
Rights and permissions
About this article
Cite this article
Cotter, G., Davison, B.A., Butler, J. et al. Relationship between baseline systolic blood pressure and long-term outcomes in acute heart failure patients treated with TRV027: an exploratory subgroup analysis of BLAST-AHF. Clin Res Cardiol 107, 170–181 (2018). https://doi.org/10.1007/s00392-017-1168-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-017-1168-0