Abstract
Background
Drug-eluting stents successfully reduce restenosis at the cost of delayed re-endothelialization. A novel concept to enhance re-endothelialization is the use of antibody-coated stents which capture circulating progenitor cells. A CD34-positive-cell-capturing stent was recently developed with conflicting clinical results. CD133 is a glycoprotein expressed on circulating hematopoietic and putative endothelial-regenerating cells and may be superior to CD34.
Objective
The aim of our study was to develop a CD133-cell-capturing bare-metal stent and investigate feasibility, safety, and efficacy of CD133-stents in terms of re-endothelialization and neointima inhibition.
Methods and results
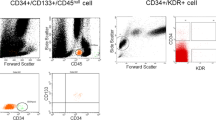
Anti-human CD133-antibodies were covalently attached to bare-metal stents. In vitro, binding capacity of CD133-stents was studied, revealing a significantly higher affinity of human CD133-positive cells to CD133-stents compared with mononuclear cells (MNCs). In vivo, 15 landrace pigs received BMS and CD133-stents in either RCX or LAD (n = 30 stents). Re-endothelialization was examined on day 1 (n = 4), 3 (n = 4) and day 7 (n = 4) using scanning electron microscopy. In histology, injury and inflammatory scores, as well as diameter restenosis were evaluated after day 7 (n = 3), 14 (n = 4), and 28 (n = 2). Overall no reduction in re-endothelialization, diameter stenosis or inflammatory score was seen with CD133-stents.
Conclusion
Stent coating with anti-human CD133-antibodies was successfully achieved with effective binding of CD133-positive cells. However, in vivo, no difference in re-endothelialization or neointima formation was evident with the use of CD133-stents compared with BMS. The low number of circulating CD133-positive cells and an increase in unspecific binding of MNCs over time may account for the observed lack of efficacy.








Similar content being viewed by others
Abbreviations
- BMS:
-
Bare metal stent
- EC:
-
Endothelial cells
- EPC:
-
Endothelial progenitor cells
- ISR:
-
Instent restenosis
- (L)ST:
-
(Late) Stent thrombosis
- MNC:
-
Mononuclear cells
- SEM:
-
Scanning electron microscopy
References
Takayama T, Hiro T, Hirayama A (2011) Stent thrombosis and drug-eluting stents. J Cardiol 58(2):92–98
Suzuki T, Kopia G, Hayashi S, Bailey LR, Llanos G, Wilensky R, Klugherz BD, Papandreou G, Narayan P, Leon MB, Yeung AC, Tio F, Tsao PS, Falotico R, Carter AJ (2001) Stent-based delivery of sirolimus reduces neointimal formation in a porcine coronary model. Circulation 104(10):1188–1193
Siller-Matula JM, Tentzeris I, Vogel B, Schacherl S, Jarai R, Geppert A, Unger G, Huber K (2010) Tacrolimus-eluting carbon-coated stents versus sirolimus-eluting stents for prevention of symptom-driven clinical end points. Clin Res Cardiol 99(10):645–650
Zahn R, Hamm CW, Zeymer U, Richardt G, Kelm M, Levenson B, Bonzel T, Tebbe U, Sabin G, Nienaber CA, Pfannebecker T, Senges J (2010) Coronary stenting with the sirolimus-eluting stent in patients with restenosis after intracoronary brachytherapy: results from the prospective multicentre German Cypher Stent Registry. Clin Res Cardiol 99(2):99–106
Klugherz BD, Llanos G, Lieuallen W, Kopia GA, Papandreou G, Narayan P, Sasseen B, Adelman SJ, Falotico R, Wilensky RL (2002) Twenty-eight-day efficacy and phamacokinetics of the sirolimus-eluting stent. Coron Artery Dis 13(3):183–188
Drachman DE, Edelman ER, Seifert P, Groothuis AR, Bornstein DA, Kamath KR, Palasis M, Yang D, Nott SH, Rogers C (2000) Neointimal thickening after stent delivery of paclitaxel: change in composition and arrest of growth over six months. J Am Coll Cardiol 36(7):2325–2332
Farb A, Heller PF, Shroff S, Cheng L, Kolodgie FD, Carter AJ, Scott DS, Froehlich J, Virmani R (2001) Pathological analysis of local delivery of paclitaxel via a polymer-coated stent. Circulation 104(4):473–479
Finn AV, Kolodgie FD, Harnek J, Guerrero LJ, Acampado E, Tefera K, Skorija K, Weber DK, Gold HK, Virmani R (2005) Differential response of delayed healing and persistent inflammation at sites of overlapping sirolimus- or paclitaxel-eluting stents. Circulation 112(2):270–278
Luscher TF, Steffel J, Eberli FR, Joner M, Nakazawa G, Tanner FC, Virmani R (2007) Drug-eluting stent and coronary thrombosis: biological mechanisms and clinical implications. Circulation 115(8):1051–1058
Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, Kutys R, Skorija K, Gold HK, Virmani R (2006) Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol 48(1):193–202
Joner M, Nakazawa G, Finn AV, Quee SC, Coleman L, Acampado E, Wilson PS, Skorija K, Cheng Q, Xu X, Gold HK, Kolodgie FD, Virmani R (2008) Endothelial cell recovery between comparator polymer-based drug-eluting stents. J Am Coll Cardiol 52(5):333–342
Zampetaki A, Kirton JP, Xu Q (2008) Vascular repair by endothelial progenitor cells. Cardiovasc Res 78(3):413–421
Volaklis KA, Tokmakidis SP, Halle M (2012) Acute and chronic effects of exercise on circulating endothelial progenitor cells in healthy and diseased patients. Clin Res Cardiol. doi:10.1007/s00392-012-0517-2
Katsuki Y, Sasaki K, Toyama Y, Ohtsuka M, Koiwaya H, Nakayoshi T, Imaizumi T (2011) Early outgrowth EPCs generation is reduced in patients with Buerger’s disease. Clin Res Cardiol 100(1):21–27
Karthikeyan VJ, Blann AD, Baghdadi S, Lane DA, Gareth BD, Lip GY (2011) Endothelial dysfunction in hypertension in pregnancy: associations between circulating endothelial cells, circulating progenitor cells and plasma von Willebrand factor. Clin Res Cardiol 100(6):531–537
Bulut D, Scheeler M, Niedballa LM, Miebach T, Mugge A (2011) Effects of immunoadsorption on endothelial function, circulating endothelial progenitor cells and circulating microparticles in patients with inflammatory dilated cardiomyopathy. Clin Res Cardiol 100(7):603–610
Walter DH, Rittig K, Bahlmann FH, Kirchmair R, Silver M, Murayama T, Nishimura H, Losordo DW, Asahara T, Isner JM (2002) Statin therapy accelerates reendothelialization: a novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cells. Circulation 105(25):3017–3024
Werner N, Junk S, Laufs U, Link A, Walenta K, Bohm M, Nickenig G (2003) Intravenous transfusion of endothelial progenitor cells reduces neointima formation after vascular injury. Circ Res 93(2):e17–e24
Hagensen MK, Raarup MK, Mortensen MB, Thim T, Nyengaard JR, Falk E, Bentzon JF (2012) Circulating endothelial progenitor cells do not contribute to regeneration of endothelium after murine arterial injury. Cardiovasc Res 93(2):223–231
Yoder MC (2009) Defining human endothelial progenitor cells. J Thromb Haemost 7(Suppl 1):49–52
Fadini GP, de Kreutzenberg SV, Coracina A, Baesso I, Agostini C, Tiengo A, Avogaro A (2006) Circulating CD34+ cells, metabolic syndrome, and cardiovascular risk. Eur Heart J 27(18):2247–2255
Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Bohm M, Nickenig G (2005) Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med 353(10):999–1007
Case J, Mead LE, Bessler WK, Prater D, White HA, Saadatzadeh MR, Bhavsar JR, Yoder MC, Haneline LS, Ingram DA (2007) Human CD34+ AC133+ VEGFR-2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Exp Hematol 35(7):1109–1118
Wendel HP, Avci-Adali M, Ziemer G (2010) Endothelial progenitor cell capture stents–hype or hope? Int J Cardiol 145(1):115–117
Co M, Tay E, Lee CH, Poh KK, Low A, Lim J, Lim IH, Lim YT, Tan HC (2008) Use of endothelial progenitor cell capture stent (Genous Bio-Engineered R Stent) during primary percutaneous coronary intervention in acute myocardial infarction: intermediate- to long-term clinical follow-up. Am Heart J 155(1):128–132
Aoki J, Serruys PW, van BH, Ong AT, McFadden EP, Sianos G, van der Giessen WJ, Regar E, de Feyter PJ, Davis HR, Rowland S, Kutryk MJ (2005) Endothelial progenitor cell capture by stents coated with antibody against CD34: the HEALING-FIM (Healthy Endothelial Accelerated Lining Inhibits Neointimal Growth-First In Man) Registry. J Am Coll Cardiol 45(10):1574–1579
Rossi ML, Zavalloni D, Gasparini GL, Mango R, Belli G, Presbitero P (2010) The first report of late stent thrombosis leading to acute myocardial infarction in patient receiving the new endothelial progenitor cell capture stent. Int J Cardiol 141(1):e20–e22
Klomp M, Beijk MA, Varma C, Koolen JJ, Teiger E, Richardt G, Bea F, Van GN, Verouden NJ, Chan YK, Woudstra P, Damman P, Tijssen JG, de Winter RJ (2011) 1-year outcome of TRIAS HR (TRI-stent adjudication study-high risk of restenosis) a multicenter, randomized trial comparing genous endothelial progenitor cell capturing stents with drug-eluting stents. JACC Cardiovasc Interv 4(8):896–904
van Beusekom HM, Ertas G, Sorop O, Serruys PW, van der Giessen WJ (2012) The genous endothelial progenitor cell capture stent accelerates stent re-endothelialization but does not affect intimal hyperplasia in porcine coronary arteries. Catheter Cardiovasc Interv 79(2):231–242
Wohrle J, Birkemeyer R, Markovic S, Nguyen TV, Sinha A, Miljak T, Spiess J, Rottbauer W, Rittger H (2011) Prospective randomised trial evaluating a paclitaxel-coated balloon in patients treated with endothelial progenitor cell capturing stents for de novo coronary artery disease. Heart 97(16):1338–1342
Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, Rafii S (2000) Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood 95(3):952–958
Stamm C, Westphal B, Kleine HD, Petzsch M, Kittner C, Klinge H, Schumichen C, Nienaber CA, Freund M, Steinhoff G (2003) Autologous bone-marrow stem-cell transplantation for myocardial regeneration. Lancet 361(9351):45–46
Urbich C, Dimmeler S (2004) Endothelial progenitor cells: characterization and role in vascular biology. Circ Res 95(4):343–353
Friedrich EB, Walenta K, Scharlau J, Nickenig G, Werner N (2006) CD34−/CD133+/VEGFR-2+ endothelial progenitor cell subpopulation with potent vasoregenerative capacities. Circ Res 98(3):e20–e25
Schwartz RS, Edelman ER, Carter A, Chronos N, Rogers C, Robinson KA, Waksman R, Weinberger J, Wilensky RL, Jensen DN, Zuckerman BD, Virmani R (2002) Drug-eluting stents in preclinical studies: recommended evaluation from a consensus group. Circulation 106(14):1867–1873
van Beusekom H, Sorop O, Weymaere M, Duncker D, van der Giessen W (2008) The neointimal response to stents eluting tacrolimus from a degradable coating depends on the balance between polymer degradation and drug release. EuroIntervention 4(1):139–147
Rippstein P, Black MK, Boivin M, Veinot JP, Ma X, Chen YX, Human P, Zilla P, O’Brien ER (2006) Comparison of processing and sectioning methodologies for arteries containing metallic stents. J Histochem Cytochem 54(6):673–681
Schwartz RS, Huber KC, Murphy JG, Edwards WD, Camrud AR, Vlietstra RE, Holmes DR (1992) Restenosis and the proportional neointimal response to coronary artery injury: results in a porcine model. J Am Coll Cardiol 19(2):267–274
Kornowski R, Hong MK, Tio FO, Bramwell O, Wu H, Leon MB (1998) In-stent restenosis: contributions of inflammatory responses and arterial injury to neointimal hyperplasia. J Am Coll Cardiol 31(1):224–230
de Prado AP, Perez-Martinez C, Cuellas-Ramon C, Gonzalo-Orden JM, Regueiro-Purrinos M, Martinez B, Garcia-Iglesias MJ, Ajenjo JM, Altonaga JR, Diego-Nieto A, de Miguel A, Fernandez-Vazquez F (2011) Time course of reendothelialization of stents in a normal coronary swine model: characterization and quantification. Vet Pathol 48(6):1109–1117
Asahara T, Bauters C, Pastore C, Kearney M, Rossow S, Bunting S, Ferrara N, Symes JF, Isner JM (1995) Local delivery of vascular endothelial growth factor accelerates reendothelialization and attenuates intimal hyperplasia in balloon-injured rat carotid artery. Circulation 91(11):2793–2801
Lim WH, Seo WW, Choe W, Kang CK, Park J, Cho HJ, Kyeong S, Hur J, Yang HM, Cho HJ, Lee YS, Kim HS (2011) Stent coated with antibody against vascular endothelial-cadherin captures endothelial progenitor cells, accelerates re-endothelialization, and reduces neointimal formation. Arterioscler Thromb Vasc Biol 31(12):2798–2805
Nakazawa G, Granada JF, Alviar CL, Tellez A, Kaluza GL, Guilhermier MY, Parker S, Rowland SM, Kolodgie FD, Leon MB, Virmani R (2010) Anti-CD34 antibodies immobilized on the surface of sirolimus-eluting stents enhance stent endothelialization. JACC Cardiovasc Interv 3(1):68–75
Nakazawa G, Finn AV, John MC, Kolodgie FD, Virmani R (2007) The significance of preclinical evaluation of sirolimus-, paclitaxel-, and zotarolimus-eluting stents. Am J Cardiol 100(8B):36M–44M
Miyauchi K, Kasai T, Yokayama T, Aihara K, Kurata T, Kajimoto K, Okazaki S, Ishiyama H, Daida H (2008) Effectiveness of statin-eluting stent on early inflammatory response and neointimal thickness in a porcine coronary model. Circ J 72(5):832–838
Suzuki T, Kopia G, Hayashi S, Bailey LR, Llanos G, Wilensky R, Klugherz BD, Papandreou G, Narayan P, Leon MB, Yeung AC, Tio F, Tsao PS, Falotico R, Carter AJ (2001) Stent-based delivery of sirolimus reduces neointimal formation in a porcine coronary model. Circulation 104(10):1188–1193
Garcia-Touchard A, Burke SE, Toner JL, Cromack K, Schwartz RS (2006) Zotarolimus-eluting stents reduce experimental coronary artery neointimal hyperplasia after 4 weeks. Eur Heart J 27(8):988–993
Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, Olweus J, Kearney J, Buck DW (1997) AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood 90(12):5002–5012
Gehling UM, Ergun S, Schumacher U, Wagener C, Pantel K, Otte M, Schuch G, Schafhausen P, Mende T, Kilic N, Kluge K, Schafer B, Hossfeld DK, Fiedler W (2000) In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood 95(10):3106–3112
Flores-Ramirez R, Uribe-Longoria A, Rangel-Fuentes MM, Gutierrez-Fajardo P, Salazar-Riojas R, Cervantes-Garcia D, Trevino-Ortiz JH, Benavides-Chereti GJ, Espinosa-Oliveros LP, Limon-Rodriguez RH, Monreal-Puente R, Gonzalez-Trevino JL, Rojas-Martinez A (2010) Intracoronary infusion of CD133+ endothelial progenitor cells improves heart function and quality of life in patients with chronic post-infarct heart insufficiency. Cardiovasc Revasc Med 11(2):72–78
Langer HF, von der Ruhr JW, Daub K, Schoenberger T, Stellos K, May AE, Schnell H, Gauss A, Hafner R, Lang P, Schumm M, Buhring HJ, Klingel K, Conrad S, Schaller M, van Zandvoort M, Jung G, Dimmeler S, Skutella T, Gawaz M (2010) Capture of endothelial progenitor cells by a bispecific protein/monoclonal antibody molecule induces reendothelialization of vascular lesions. J Mol Med (Berl) 88(7):687–699
Case J, Mead LE, Bessler WK, Prater D, White HA, Saadatzadeh MR, Bhavsar JR, Yoder MC, Haneline LS, Ingram DA (2007) Human CD34+ AC133+ VEGFR-2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Exp Hematol 35(7):1109–1118
Blyszczuk P, Germano D, Stein S, Moch H, Matter CM, Beck-Schimmer B, Luscher TF, Eriksson U, Kania G (2011) Profibrotic potential of prominin-1(+) epithelial progenitor cells in pulmonary fibrosis. Respir Res 12:126
Jaszai J, Fargeas CA, Florek M, Huttner WB, Corbeil D (2007) Focus on molecules: prominin-1 (CD133). Exp Eye Res 85(5):585–586
Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S (2001) Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res 89(1):E1–E7
Wang TJ, Yang YJ, Xu B, Zhang Q, Jin C, Tang Y, Tian Y, Mintz GS (2012) Atorvastatin accelerates both neointimal coverage and re-endothelialization after sirolimus-eluting stent implantation in a porcine model: new findings from optical coherence tomography and pathology. Circ J 76(11):2561–2571
Dzau VJ, Gnecchi M, Pachori AS, Morello F, Melo LG (2005) Therapeutic potential of endothelial progenitor cells in cardiovascular diseases. Hypertension 46(1):7–18
Timmermans F, Van HF, De SM, Raedt R, Plasschaert F, De Buyzere ML, Gillebert TC, Plum J, Vandekerckhove B (2007) Endothelial outgrowth cells are not derived from CD133+ cells or CD45+ hematopoietic precursors. Arterioscler Thromb Vasc Biol 27(7):1572–1579
Richardson MR, Yoder MC (2011) Endothelial progenitor cells: quo vadis? J Mol Cell Cardiol 50(2):266–272
Estes ML, Mund JA, Ingram DA, Case J (2010) Identification of endothelial cells and progenitor cell subsets in human peripheral blood. Curr Protoc Cytom Chapter 9:Unit-11
Silber S, Damman P, Klomp M, Beijk MA, Grisold M, Ribeiro EE, Suryapranata H, Wojcik J, Hian SK, Tijssen JG, de Winter RJ (2011) Clinical results after coronary stenting with the Genous Bio-engineered R stent: 12-month outcomes of the e-HEALING (Healthy Endothelial Accelerated Lining Inhibits Neointimal Growth) worldwide registry. EuroIntervention 6(7):819–825
Wohrle J, Rottbauer W, Imhof A (2012) Everolimus-eluting stents for treatment of chronic total coronary occlusions. Clin Res Cardiol 101(1):23–28
Zahn R, Ischinger T, Zeymer U, Brachmann J, Jung J, Haase H, Hauptmann KE, Seggewiss H, Janicke I, Leschke M, Mudra H (2010) Carotid artery interventions for restenosis after prior stenting: is it different from interventions of de novo lesions? Results from the carotid artery stent (CAS)–registry of the Arbeitsgemeinschaft Leitende Kardiologische Krankenhausarzte (ALKK). Clin Res Cardiol 99(12):809–815
Cremers B, Toner JL, Schwartz LB, von OR, Speck U, Kaufels N, Clever YP, Mahnkopf D, Bohm M, Scheller B (2012) Inhibition of neointimal hyperplasia with a novel zotarolimus coated balloon catheter. Clin Res Cardiol 101(6):469–476
Unverdorben M, Kleber FX, Heuer H, Figulla HR, Vallbracht C, Leschke M, Cremers B, Hardt S, Buerke M, Ackermann H, Boxberger M, Degenhardt R, Scheller B (2010) Treatment of small coronary arteries with a paclitaxel-coated balloon catheter. Clin Res Cardiol 99(3):165–174
Shen L, Wu Y, Zhang F, Wu L, Dong C, Gao Y, Sun A, Zou Y, Qian J, Sun J, Zhong W, Ge J (2012) Assessment of an asymmetrical coating stent with sirolimus released from ablumial matrix in porcine model. Clin Res Cardiol 101(11):917–927
Acknowledgments
The authors would like to thank Andrea and Ute Lohmer for their excellent support in this study. The Prokinetic Energy stent was provided by Biotronik GmbH (Berlin, Germany). The sponsor had no influence on either the conducting or the evaluation of this study.
Conflict of interest
There are no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sedaghat, A., Sinning, JM., Paul, K. et al. First in vitro and in vivo results of an anti-human CD133-antibody coated coronary stent in the porcine model. Clin Res Cardiol 102, 413–425 (2013). https://doi.org/10.1007/s00392-013-0547-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-013-0547-4