Abstract
Purpose
Pharmacologic modulation of the perioperative physiologic stress response, using the beta-blocker propranolol, combined with the COX-2 inhibitor etodolac, has been shown to reduce metastatic spread and increase survival rates following surgery for primary tumor excision in rodents. Prior to implantation of this pharmacological approach in clinical trials in patients with colon cancer, the safety of this technique has to be evaluated. This study assessed the effects of these drugs on the healing of colonic anastomosis in rats.
Methods
Forty-eight F344 rats were divided into two groups, which were given seven daily subcutaneous injections of either vehicle, or propranolol (up to 1.2 mg/kg/day) combined with etodolac (12.5 mg/kg/day), starting the day before surgery. Each animal underwent laparotomy, colotomy of the descending colon, and anastomosis. Anastomotic leak rate and bursting pressure were compared at 1 week after the operation. The harvested anastomosis was histologically assessed for wound healing parameters.
Results
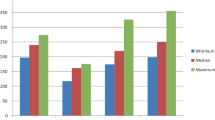
Forty-three rats survived the operation and were eligible for analysis at 1 week. No significant difference in survival, anastomotic leakage, or bursting pressure was found between animals that received propranolol and etodolac versus those receiving vehicle (drugs 179 mmHg ± 45.4; vehicle 187 mmHg, SD ± 35.0, p = 0.54). Histologic assessment of fibrosis, necrosis, cell infiltration, and tissue reaction zone did not differ between the two groups.
Conclusions
Perioperative administration of propranolol and etodolac seems safe in colon operations in rats and does not affect anastomotic failure or colon healing.
Similar content being viewed by others
References
Shakhar G, Ben-Eliyahu S (2003) Potential prophylactic measures against postoperative immunosuppression: could they reduce recurrence rates in oncological patients? Ann Surg Oncol 10:972–992
Lutgendorf SK, Cole S, Costanzo E, Bradley S, Coffin J, Jabbari S, Rainwater K, Ritchie JM, Yang M, Sood AK (2003) Stress-related mediators stimulate vascular endothelial growth factor secretion by two ovarian cancer cell lines. Clin Cancer Res 9:4514–4521
Wojtowicz-Praga S (1997) Reversal of tumor-induced immunosuppression: a new approach to cancer therapy. J Immunother 20:165–177
Oosterling SJ, van der Bij GJ, van Egmond M, van der Sijp JR (2005) Surgical trauma and peritoneal recurrence of colorectal carcinoma. Eur J Surg Oncol 31:29–37
van der Bij GJ, Oosterling SJ, Beelen RH, Meijer S, Coffey JC, van Egmond M (2009) The perioperative period is an underutilized window of therapeutic opportunity in patients with colorectal cancer. Ann Surg 249:727–734
Zetter BR (1998) Angiogenesis and tumor metastasis. Annu Rev Med 49:407–424
Kern MA, Haugg AM, Koch AF, Schilling T, Breuhahn K, Walczak H et al (2006) Cyclooxygenase-2 inhibition induces apoptosis signaling via death receptors and mitochondria in hepatocellular carcinoma. Cancer Res 66:7059–7066
Wei D, Wang L, He Y et al (2004) Celecoxib inhibits vascular endothelial growth factor expression in and reduces angiogenesis and metastasis of human pancreatic cancer via suppression of Sp1 transcription factor activity. Cancer Res 64:2030–2038
Svane IM, Boesen M, Engel AM (1999) The role of cytotoxic T-lymphocytes in the prevention and immune surveillance of tumors—lessons from normal and immunodeficient mice. Med Oncol 16:223–38
van Egmond M (2008) Neutrophils in antibody-based immunotherapy of cancer. Expert Opin Biol Ther 8:83–94
van der Bij GJ, Oosterling SJ, Meijer S, Beelen RH, van Egmond M (2005) The role of macrophages in tumor development. Cell Oncol 27:203–213
Rozic JG, Chakraborty C, Lala PK (2001) Cyclooxygenase inhibitors retard murine mammary tumor progression by reducing tumor cell migration, invasiveness and angiogenesis. Int J Cancer 93:497–506
Thaker PH, Han LY, Kamat AA et al (2006) Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med 12:939–944
Masur K, Niggemann B, Zanker KS et al (2001) Norepinephrine induced migration of SW 480 colon carcinoma cells is inhibited by beta-blockers. Cancer Res 61:2866–2869
Sood AK, Bhatty R, Kamat AA et al (2006) Stress hormone-mediated invasion of ovarian cancer cells. Clin Cancer Res 12:369–375
Melamed R, Rosenne E, Shakhar K et al (2005) Marginating pulmonary-NK activity and resistance to experimental tumor metastasis: suppression by surgery and the prophylactic use of a beta-adrenergic antagonist and a prostaglandin synthesis inhibitor. Brain Behav Immun 19:114–126
Benish M, Bartal I, Goldfarb Y et al (2008) Perioperative use of beta-blockers and cyclooxygenase-2 inhibitors may improve immune competence and reduce the risk of tumor metastasis. Ann Surg Oncol 15:2042–2052
Glasner A, Avraham R, Rosenne E, Benish M, Zmora O, Shemer S, Meiboom H, Ben-Eliyahu S (2010) Improving survival rates in two models of spontaneous postoperative metastasis in mice by combined administration of a beta-adrenergic antagonist and a cyclooxygenase-2 inhibitor. J Immunol 184:2449–2457
Dvorak HF (1986) Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 315:1650–1659
Shi JM, Lai SG, Xu CJ et al (2004) Pharmacokinetic difference between S-(+)- and R-(−)-etodolac in rats. Acta Pharmacol Sin 25:996–999
Barnes PJ, Adcock I (1993) Anti-inflammatory actions of steroids: molecular mechanisms. Trends Pharmacol Sci 14:436–441
Eschwege P, Dumas F, Blanchet P, Le Maire V, Benoit G, Jardin A, Lacour B, Loric S (1995) Haematogenous dissemination of prostatic epithelial cells during radical prostatectomy. Lancet 346(8989):1528–1530
Yamaguchi K, Takagi Y, Aoki S, Saji S (2000) Significant detection of circulating cancer cells in the blood by reverse transcriptase-polymerase chain reaction during colorectal cancer resection. Ann Surg 232:58–65
Ben-Eliyahu S (1998) Stress, natural killer cell activity, and tumor metastasis: the role of catecholamines and corticosteroids. In: Levi A et al (eds) New frontiers in stress research: modulation in brain function. Harwood Academic Publishers GmbH, Amsterdam, pp 203–215
Page GG, Ben-Eliyahu S, Liebeskind JC (1994) The role of LGL/NK cells in surgery-induced promotion of metastasis and its attenuation by morphine. Brain Behav Immun 8:241–250
Thompson SD, Chang EU, Jobe BA (2006) Clinical review: healing in gastrointestinal anastomoses. Microsurgery 26:131–136
Cahill RA, Sheehan KM, Scanlon RW et al (2004) Effects of COX-2 inhibitor on colonic anastomotic and skin wound integrity. Br J Surg 12:1613–1618
de Hingh IH, van Goor H, de Man BM et al (2006) Selective COX-2 inhibitors impair ileal but not colonic experimental anastomotic healing in the early postoperative period. Br J Surg 4:489–497
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Benjamin, B., Hazut, O., Shaashua, L. et al. Effect of beta blocker combined with COX-2 inhibitor on colonic anastomosis in rats. Int J Colorectal Dis 25, 1459–1464 (2010). https://doi.org/10.1007/s00384-010-0992-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-010-0992-8