Abstract
Purpose
Necrotizing enterocolitis (NEC) is a gastrointestinal disease in neonates that is associated with immune-mediated intestinal inflammation. Remote ischemic conditioning (RIC) applied to a limb has been shown to be protective against experimental NEC. In this study, we explore the immune cell-mediated response involved in NEC and the immunomodulatory effects of RIC in an experimental mouse model of the disease.
Methods
NEC was induced in C57BL/6 mice (ethical approval #58119) pups on postnatal day5 (p5) using gavage hyperosmolar formula, lipopolysaccharide, and hypoxia. RIC consisted of 4 cycles of 5 min ischemia followed by 5 min reperfusion of the right hindlimb during NEC induction on p6 and p8. Breastfed mice were used as control. The mice were sacrificed on p9, with ileal tissue evaluated for inflammatory cytokines and by characterization of T-cell populations.
Results
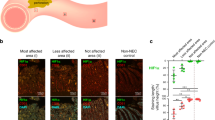
NEC mice had increased number of CD4+ cells indicating an accumulation of T-cells in the mesenchyme of the NEC ileum. Compared to control, NEC pups had upregulated expression pro-inflammatory cytokines (GATA3, IFNγ, IL1β, IL6, IL17, IL22, and TNFα) and reduced anti-inflammatory cytokine (TGFβ). In NEC, there was also a shift in the balance of Treg/Th17 cells towards Th17. Compared to NEC alone, RIC during the course of NEC resulted in reduction of pro-inflammatory cytokines (GATA3, IFNγ, IL1β, IL6, IL17, IL22, and TNFα), increase in anti-inflammatory cytokine TGFβ and concomitant shift back of Th17 cells towards Treg cells.
Conclusion
In experimental NEC, remote ischemic conditioning reduces the production of pro-inflammatory markers and increases the production of anti-inflammatory markers. In addition, during NEC, RIC reverses the imbalance of Treg/Th17 providing support for its effect on cell-mediated inflammation. RIC is a non-invasive physical maneuver that can have a significant beneficial effect in reducing the inflammation seen in NEC.




Similar content being viewed by others
References
Neu J, Walker WA (2011) Necrotizing enterocolitis. N Engl J Med 364(3):255–264. https://doi.org/10.1056/NEJMra1005408
Thompson AM, Bizzarro MJ (2008) Necrotizing enterocolitis in newborns: pathogenesis, prevention and management. Drugs 68(9):1227–1238. https://doi.org/10.2165/00003495-200868090-00004
Alganabi M, Lee C, Bindi E, Li B, Pierro A (2019) Recent advances in understanding necrotizing enterocolitis. F1000Res J 8:F1000. https://doi.org/10.1268/f1000research.17228.1
Zhang C, Sherman MP, Prince LS, Bader D, Weitkamp JH, Slaughter JC et al (2012) Paneth cell ablation in the presence of Klebsiella pneumoniae induces necrotizing enterocolitis (NEC)-like injury in the small intestine of immature mice. Dis Model Mech 5(4):522–532. https://doi.org/10.1242/dmm.009001
Huda S, Chaudhery S, Ibrahim H, Pramanik A (2014) Neonatal necrotizing enterocolitis: clinical challenges, pathophysiology and management. Pathophysiology 21(1):3–12. https://doi.org/10.1016/j.pathophys.2013.11.009
Bazacliu C, Neu J (2019) Necrotizing enterocolitis: long term complications. Curr Pediatr Rev 15(2):115–124. https://doi.org/10.2174/1573396315666190312093119
Lim JC, Golden JM, Ford HR (2015) Pathogenesis of neonatal necrotizing enterocolitis. Pediatr Surg Int 31(6):509–518. https://doi.org/10.1007/s00383-015-3697-9
Lu P, Sodhi CP, Hackam DJ (2014) Toll-like receptor regulation of intestinal development and inflammation in the pathogenesis of necrotizing enterocolitis. Pathophysiology 21(1):81–93. https://doi.org/10.1016/j.pathophys.2013.11.007
Besner GE (2015) A pain in the NEC: research challenges and opportunities. J Pediatr Surg 50(1):23–29. https://doi.org/10.1016/j.jpedsurg.2014.10.024
Patel RM, Denning PW (2015) Intestinal microbiota and its relationship with necrotizing enterocolitis. Pediatr Res 78(3):232–238. https://doi.org/10.1038/pr.2015.97
Yikilmaz A, Hall NJ, Daneman A, Gerstle JT, Navarro OM, Moineddin R et al (2014) Prospective evaluation of the impact of sonography on the management and surgical intervention of neonates with necrotizing enterocolitis. Pediatr Surg Int 30(12):1231–1240
Su Y, Besner GE (2014) Heparin-binding EGF-like growth factor (HB-EGF) promotes cell migration and adhesion via focal adhesion kinase. J Surg Res 189(2):222–231. https://doi.org/10.1016/j.jss.2014.02.055
Takeda K, Akira S (2004) TLR signaling pathways. Semin Immunol 16(1):3–9. https://doi.org/10.1016/j.smim.2003.10.003
Lu YC, Yeh WC, Ohashi PS (2008) LPS/TLR4 signal transduction pathway. Cytokine 42(2):145–151. https://doi.org/10.1016/j.cyto.2008.01.006
Leaphart CL, Cavallo J, Gribar SC, Cetin S, Li J, Branca MF et al (2007) A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J Immunol 179(7):4808–4820. https://doi.org/10.4049/jimmunol.179.7.4808
Egan CE, Sodhi CP, Good M, Lin J, Jia H, Yamaguchi Y et al (2016) Toll-like receptor 4-mediated lymphocyte influx induces neonatal necrotizing enterocolitis. J Clin Invest 126(2):495–508. https://doi.org/10.1172/JCI83356
Murry CE, Jennings RB, Reimer KA (1986) Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 74(5):1124–1136. https://doi.org/10.1161/01.cir.74.5.1124
Lim SY, Hausenloy DJ (2012) Remote ischemic conditioning: from bench to bedside. Front Physiol 3:27. https://doi.org/10.3389/fphys.2012.00027
Koike Y, Li B, Ganji N, Zhu H, Miyake H, Chen Y et al (2020) Remote ischemic conditioning counteracts the intestinal damage of necrotizing enterocolitis by improving intestinal microcirculation. Nat Commun 11(1):4950. https://doi.org/10.1038/s41467-020-18750-9
Yang J, Shakil F, Cho S (2019) Peripheral mechanisms of remote ischemic conditioning. Cond Med 2(2):61–68
Kim YH, Yoon DW, Kim JH, Lee JH, Lim CH (2014) Effect of remote ischemic post-conditioning on systemic inflammatory response and survival rate in lipopolysaccharide-induced systemic inflammation model. J Inflamm (Lond) 11:16. https://doi.org/10.1186/1476-9255-11-16
Zhou X, Jiang R, Dong Y, Wang L (2017) Remote ischemic preconditioning attenuates cardiopulmonary bypass-induced lung injury. PLoS One 12(12):e0189501. https://doi.org/10.1371/journal.pone.0189501
Konstantinov IE, Arab S, Kharbanda RK, Li J, Cheung MM, Cherepanov V et al (2004) The remote ischemic preconditioning stimulus modifies inflammatory gene expression in humans. Physiol Genomics 19(1):143–150. https://doi.org/10.1152/physiolgenomics.00046.2004
Yamoto M, Alganabi M, Chusilp S, Lee D, Yazaki Y, Lee C et al (2020) Lysosomal overloading and necrotizing enterocolitis. Pediatr Surg Int 36(10):1157–1165. https://doi.org/10.1007/s00383-020-04724-x
Li B, Wu RY, Horne RG, Ahmed A, Lee D, Robinson SC et al (2020) Human milk oligosaccharides protect against necrotizing enterocolitis by activating intestinal cell differentiation. Mol Nutr Food Res 64(21):e2000519. https://doi.org/10.1002/mnfr.202000519
Miyake H, Lee C, Chusilp S, Bhalla M, Li B, Pitino M et al (2020) Human breast milk exosomes attenuate intestinal damage. Pediatr Surg Int 36(2):155–163. https://doi.org/10.1007/s00383-019-04599-7
Miyake H, Chen Y, Koike Y, Hock A, Li B, Lee C et al (2016) Osmolality of enteral formula and severity of experimental necrotizing enterocolitis. Pediatr Surg Int 32(12):1153–1156. https://doi.org/10.1007/s00383-016-3998-7
Chen Y, Koike Y, Miyake H, Li B, Lee C, Hock A et al (2016) Formula feeding and systemic hypoxia synergistically induce intestinal hypoxia in experimental necrotizing enterocolitis. Pediatr Surg Int 32(12):1115–1119
Zani A, Zani-Ruttenstock E, Peyvandi F, Lee C, Li B, Pierro A (2016) A spectrum of intestinal injury models in neonatal mice. Pediatr Surg Int 32(1):65–70. https://doi.org/10.1007/s00383-015-3813-x
Yang J, Shakil F, Cho S (2019) Peripheral mechanisms of remote ischemic conditioning. Cond Med 2:61–68
Waite JC, Skokos D (2012) Th17 response and inflammatory autoimmune diseases. Int J Inflam 2012:819467. https://doi.org/10.1155/2012/819467
Yan JB, Luo MM, Chen ZY, He BH (2020) The function and role of the Th17/Treg cell balance in inflammatory bowel disease. J Immunol Res 2020:8813558. https://doi.org/10.1155/2020/8813558
Shao S, Yu X, Shen L (2018) Autoimmune thyroid diseases and Th17/Treg lymphocytes. Life Sci 192:160–165. https://doi.org/10.1016/j.lfs.2017.11.026
Shan J, Jin H, Xu Y (2020) T cell metabolism: a new perspective on Th17/Treg cell imbalance in systemic lupus erythematosus. Front Immunol 11:1027. https://doi.org/10.3389/fimmu.2020.01027
Lee GR (2018) The balance of Th17 versus Treg cells in autoimmunity. Int J Mol Sci 19(3):730. https://doi.org/10.3390/ijms19030730.PMID:29510522;PMCID:PMC5877591
Han L, Yang J, Wang X, Li D, Lv L, Li B (2015) Th17 cells in autoimmune diseases. Front Med 9(1):10–19. https://doi.org/10.1007/s11684-015-0388-9
Beringer A, Noack M, Miossec P (2016) IL-17 in chronic inflammation: from discovery to targeting. Trends Mol Med 22(3):230–241. https://doi.org/10.1016/j.molmed.2016.01.001
Ma F, Li S, Gao X, Zhou J, Zhu X, Wang D et al (2019) Interleukin-6-mediated CCR9+ interleukin-17-producing regulatory T cells polarization increases the severity of necrotizing enterocolitis. EBioMedicine 44:71–85. https://doi.org/10.1016/j.ebiom.2019.05.042
Egan CE, Sodhi CP, Good M, Lin J, Jia H, Yamaguchi Y et al (2016) Toll-like receptor 4-mediated lymphocyte influx induces neonatal necrotizing enterocolitis. J Clin Invest 126(2):495–508. https://doi.org/10.1172/JCI83356
Weitkamp JH, Koyama T, Rock MT, Correa H, Goettel JA, Matta P et al (2013) Necrotising enterocolitis is characterised by disrupted immune regulation and diminished mucosal regulatory (FOXP3)/effector (CD4, CD8) T cell ratios. Gut 62(1):73–82. https://doi.org/10.1136/gutjnl-2011-301551
Dingle BM, Liu Y, Fatheree NY, Min J, Rhoads JM, Tran DQ (2013) FoxP3+ regulatory T cells attenuate experimental necrotizing enterocolitis. PLoS One 8(12):e82963. https://doi.org/10.1371/journal.pone.0082963
Lau E, Lee C, Li B, Pierro A (2021) Endoplasmic reticulum stress in the acute intestinal epithelial injury of necrotizing enterocolitis. Pediatr Surg Int 37(9):1151–1160. https://doi.org/10.1007/s00383-021-04929-8
Hamner MA, Ye Z, Lee RV, Colman JR, Le T, Gong DC et al (2015) Ischemic preconditioning in white matter: magnitude and mechanism. J Neurosci 35(47):15599–15611. https://doi.org/10.1523/JNEUROSCI.2544-15.2015
Pradillo JM, Fernández-López D, García-Yébenes I, Sobrado M, Hurtado O, Moro MA et al (2009) Toll-like receptor 4 is involved in neuroprotection afforded by ischemic preconditioning. J Neurochem 109(1):287–294. https://doi.org/10.1111/j.1471-4159.2009.05972.x
Gliem M, Krammes K, Liaw L, van Rooijen N, Hartung HP, Jander S (2015) Macrophage-derived osteopontin induces reactive astrocyte polarization and promotes re-establishment of the blood brain barrier after ischemic stroke. Glia 63(12):2198–2207. https://doi.org/10.1002/glia.22885
Ejlerskov P, Hultberg JG, Wang J, Carlsson R, Ambjørn M, Kuss M et al (2015) Lack of neuronal IFN-β-IFNAR causes lewy body- and Parkinson’s disease-like dementia. Cell 163(2):324–339. https://doi.org/10.1016/j.cell.2015.08.069
Marsh B, Stevens SL, Packard AE, Gopalan B, Hunter B, Leung PY et al (2009) Systemic lipopolysaccharide protects the brain from ischemic injury by reprogramming the response of the brain to stroke: a critical role for IRF3. J Neurosci 29(31):9839–9849. https://doi.org/10.1523/JNEUROSCI.2496-09.2009
Stevens SL, Leung PY, Vartanian KB, Gopalan B, Yang T, Simon RP et al (2011) Multiple preconditioning paradigms converge on interferon regulatory factor-dependent signaling to promote tolerance to ischemic brain injury. J Neurosci 31(23):8456–8463. https://doi.org/10.1523/JNEUROSCI.0821-11.2011
Gao Y, Ren C, Li X, Yu W, Li S, Li H et al (2021) Ischemic conditioning ameliorated hypertension and vascular remodeling of spontaneously hypertensive rat via inflammatory regulation. Aging Dis 12(1):116–131. https://doi.org/10.14336/AD.2020.0320
Alganabi M, Zhu H, O’Connell JS, Biouss G, Zito A, Li B et al (2020) Calcium/calmodulin-dependent protein kinase IV signaling pathway is upregulated in experimental necrotizing enterocolitis. Pediatr Surg Int 36(3):271–277. https://doi.org/10.1007/s00383-019-04615-w
Zhu H, Li B, Bindi E, Lee C, Alganabi M, Lok MJ et al (2021) Remote ischemic conditioning avoids the development of intestinal damage after ischemia reperfusion by reducing intestinal inflammation and increasing intestinal regeneration. Pediatr Surg Int 37(3):333–337. https://doi.org/10.1007/s00383-020-04831-9
O’Connell JS, Lee C, Farhat N, Antounians L, Zani A, Li B et al (2021) Administration of extracellular vesicles derived from human amniotic fluid stem cells: a new treatment for necrotizing enterocolitis. Pediatr Surg Int 37(3):301–309
Acknowledgements
AP is the recipient of a Canadian Institutes of Health Research (CIHR) Foundation Grant 353857. The funding had no impact on study design, data collection, analysis, interpretation, the writing of the report, or the decision to submit the paper for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Ethical approval was obtained from the ethical review board at The Hospital for Sick Children (#58119).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alganabi, M., Biouss, G., Ganji, N. et al. Remote ischemic conditioning causes CD4 T cells shift towards reduced cell-mediated inflammation. Pediatr Surg Int 38, 657–664 (2022). https://doi.org/10.1007/s00383-022-05093-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-022-05093-3