Abstract
Introduction
Multiple genetic and epigenetic factors involved in central nervous system (CNS) development influence the incidence of neural tube defects (NTDs).
Discussion
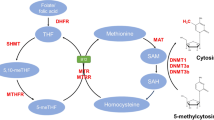
The beneficial effect of periconceptional folic acid on NTD prevention denotes a vital role for the single-carbon biochemical pathway in NTD genesis. Indeed, NTDs are associated with polymorphisms in a diversity of genes that encode folate pathway enzymes. Recent evidence suggests that CNS development and function, and consequently NTDs, are also associated with epigenetic mechanisms, many of which participate in the folate cycle and its input and output pathways. We provide an overview with select examples drawn from the authors’ research.
Similar content being viewed by others
References
Smithells RW, Sheppard S, Schorah CJ, Seller MJ, Nevin NC, Harris R, Read AP, Fielding DW (1980) Possible prevention of neural-tube defects by periconceptional vitamin supplementation. Lancet 1:339–340
Anonymous (1991) Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. MRC Vitamin Study Research Group. Lancet 338:131–137
(1992) Recommendations for the use of folic acid to reduce the number of cases of spina bifida and other neural tube defects. MMWR Morb Mortal Wkly Rep 41(RR-14):1–7
Czeizel AE, Dudas I (1992) Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med 327:1832–1835
Cornel MC, Erickson JD (1997) Comparison of national policies on periconceptional use of folic acid to prevent spina bifida and anencephaly (SBA). Teratology 55:134–137
Berry RJ, Crider KS, Yeargin-Allsopp M (2013) Periconceptional folic acid and risk of autism spectrum disorders. JAMA 309:611–613
De Wals P, Tairou F, Van Allen MI, Uh SH, Lowry RB, Sibbald B, Evans JA, Van den Hof MC, Zimmer P, Crowley M, Fernandez B, Lee NS, Niyonsenga T (2007) Reduction in neural-tube defects after folic acid fortification in Canada. N Engl J Med 357:135–142
Chen LT, Rivera MA (2004) The Costa Rican experience: reduction of neural tube defects following food fortification programs. Nutr Rev 62:S40–43
Honein MA, Paulozzi LJ, Mathews TJ, Erickson JD, Wong LY (2001) Impact of folic acid fortification of the US food supply on the occurrence of neural tube defects. JAMA 285:2981–2986
Grosse SD, Waitzman NJ, Romano PS, Mulinare J (2005) Reevaluating the benefits of folic acid fortification in the United States: economic analysis, regulation, and public health. Am J Public Health 95:1917–1922
Lopez-Camelo JS, Orioli IM, da Graca DM, Nazer-Herrera J, Rivera N, Ojeda ME, Canessa A, Wettig E, Fontannaz AM, Mellado C, Castilla EE (2005) Reduction of birth prevalence rates of neural tube defects after folic acid fortification in Chile. Am J Med Genet A 135:120–125
Hernandez-Diaz S, Werler MM, Walker AM, Mitchell AA (2000) Folic acid antagonists during pregnancy and the risk of birth defects. N Engl J Med 343:1608–1614
Nelson EW, Crick WF, Cerda JJ, Wilder BJ, Streiff RR (1983) The effect of diphenylhydantoin (phenytoin) on the sequential stages of intestinal folate absorption. Drug Nutr Interact 2:47–56
Koblin DD, Tomerson BW, Waldman FM (1990) Disruption of folate and vitamin B12 metabolism in aged rats following exposure to nitrous oxide. Anesthesiology 73:506–512
Selzer RR, Rosenblatt DS, Laxova R, Hogan K (2003) Adverse effect of nitrous oxide in a child with 5,10-methylenetetrahydrofolate reductase deficiency. N Engl J Med 349:45–50
Faessel HM, Slocum HK, Jackson RC, Boritzki TJ, Rustum YM, Nair MG, Greco WR (1998) Super in vitro synergy between inhibitors of dihydrofolate reductase and inhibitors of other folate-requiring enzymes: the critical role of polyglutamylation. Cancer Res 58:3036–3050
Quinlivan EP, McPartlin J, Weir DG, Scott J (2000) Mechanism of the antimicrobial drug trimethoprim revisited. FASEB J 14:2519–2524
Thomas DM, Zalcberg JR (1998) 5-Fluorouracil: a pharmacological paradigm in the use of cytotoxics. Clin Exp Pharmacol Physiol 25:887–895
Ullman B, Lee M, Martin DW Jr, Santi DV (1978) Cytotoxicity of 5-fluoro-2′-deoxyuridine: requirement for reduced folate cofactors and antagonism by methotrexate. Proc Natl Acad Sci U S A 75:980–983
Rosenblatt DS, Fenton WA (2000) Inherited disorders of folate and cobalamine transport and metabolism. In: Scriver CR, Sly WS, Childs B, Beaudet A, Valle D, Kinzler K, Vogelstein B (eds) The metabolic and molecular bases of inherited disease, 8th edn. McGraw-Hill, New York, pp 3897–3927
Van den Veyver IB (2002) Genetic effects of methylation diets. Annu Rev Nutr 22:255–282
Post WS, Goldschmidt-Clermont PJ, Wilhide CC, Heldman AW, Sussman MS, Ouyang P, Milliken EE, Issa JP (1999) Methylation of the estrogen receptor gene is associated with aging and atherosclerosis in the cardiovascular system. Cardiovasc Res 43:985–991
Hobbs CA, Sherman SL, Yi P, Hopkins SE, Torfs CP, Hine RJ, Pogribna M, Rozen R, James SJ (2000) Polymorphisms in genes involved in folate metabolism as maternal risk factors for Down syndrome. Am J Hum Gen 67:623–630
Roth TL (2012) Epigenetics of neurobiology and behavior during development and adulthood. Dev Psychobiol 54:590–597
Ichi S, Costa FF, Bischof JM, Nakazaki H, Shen YW, Boshnjaku V, Sharma S, Mania-Farnell B, McLone DG, Tomita T, Soares MB, Mayanil CS (2010) Folic acid remodels chromatin on Hes1 and Neurog2 promoters during caudal neural tube development. J Biol Chem 285:36922–36932
Mayanil CS, Ichi S, Farnell BM, Boshnjaku V, Tomita T, McLone DG (2011) Maternal intake of folic acid and neural crest stem cells. Vitam Horm 87:143–173
Iskandar BJ, Nelson A, Resnick D, Pate Skene JH, Gao P, Johnson C, Cook TD, Hariharan N (2004) Folic acid supplementation enhances repair of the adult central nervous system. Ann Neurol 56:221–227
Iskandar BJ, Rizk E, Meier B, Hariharan N, Bottiglieri T, Finnell RH, Jarrard DF, Banerjee RV, Skene JH, Nelson A, Patel N, Gherasim C, Simon K, Cook TD, Hogan KJ (2010) Folate regulation of axonal regeneration in the rodent central nervous system through DNA methylation. J Clin Invest 120:1603–1616
Askari BS, Krajinovic M (2010) Dihydrofolate reductase gene variations in susceptibility to disease and treatment outcomes. Current genomics 11:578–583
Boyles AL, Hammock P, Speer MC (2005) Candidate gene analysis in human neural tube defects. Am J Med Part C Sem Med Genet 135C:9–23
Zhu H, Wicker NJ, Shaw GM, Lammer EJ, Hendricks K, Suarez L, Canfield M, Finnell RH (2003) Homocysteine remethylation enzyme polymorphisms and increased risks for neural tube defects. Mol Genet Metab 78:216–221
Fagiolini M, Jensen CL, Champagne FA (2009) Epigenetic influences on brain development and plasticity. Curr Opin Neurobiol 19:207–212
Bohacek J, Mansuy IM (2013) Epigenetic inheritance of disease and disease risk. Neuropsychopharmacology 38:220–236
Mazzio EA, Soliman KF (2012) Basic concepts of epigenetics: impact of environmental signals on gene expression. Epigenetics 7:119–130
Kucharski R, Maleszka J, Foret S, Maleszka R (2008) Nutritional control of reproductive status in honeybees via DNA methylation. Science 319:1827–1830
Feng J, Chang H, Li E, Fan G (2005) Dynamic expression of de novo DNA methyltransferases Dnmt3a and Dnmt3b in the central nervous system. J Neurosci Res 79:734–746
Di Vinci A, Brigati C, Casciano I, Banelli B, Borzi L, Forlani A, Ravetti GL, Allemanni G, Melloni I, Zona G, Spaziante R, Merlo DF, Romani M (2012) HOXA7, 9, and 10 are methylation targets associated with aggressive behavior in meningiomas. Transl Res 160:355–362
Bonasio R (2012) Emerging topics in epigenetics: ants, brains, and noncoding RNAs. Ann N Y Acad Sci 1260:14–23
Harkany T (2010) HDAC9 links epigenetics to dendrite development (commentary on Sugo et al.). Eur J Neurosci 31:1519–1520
Nelson ED, Monteggia LM (2011) Epigenetics in the mature mammalian brain: effects on behavior and synaptic transmission. Neurobiol Learn Mem 96:53–60
Yao ZG, Liu Y, Zhang L, Huang L, Ma CM, Xu YF, Zhu H, Qin C (2012) Co-location of HDAC2 and insulin signaling components in the adult mouse hippocampus. Cell Mol Neurobiol 32:1337–1342
Feng J, Zhou Y, Campbell SL, Le T, Li E, Sweatt JD, Silva AJ, Fan G (2010) Dnmt1 and Dnmt3a maintain DNA methylation and regulate synaptic function in adult forebrain neurons. Nat Neurosci 13:423–430
He R, Eggert JA (2012) The finger of an angel: memory return with epigenetic manipulation. Epigenomics 4:295–302
Auger AP, Auger CJ (2011) Epigenetic turn ons and turn offs: chromatin reorganization and brain differentiation. Endocrinology 152:349–353
Szyf M (2012) Mind-body interrelationship in DNA methylation. Chemical Immunology and Allergy 98:85–99
Keverne EB (2011) Epigenetics and brain evolution. Epigenomics 3:183–191
Kosik KS, Rapp PR, Raz N, Small SA, Sweatt JD, Tsai LH (2012) Mechanisms of age-related cognitive change and targets for intervention: epigenetics. J Gerontol 67:741–746
Qureshi IA, Mehler MF (2010) The emerging role of epigenetics in stroke: II. RNA regulatory circuitry. Arc Neurol 67:1435–1441
Kurtuncu M, Tuzun E (2008) Multiple sclerosis: could it be an epigenetic disease? Medical Hypotheses 71:945–947
Chestnut BA, Chang Q, Price A, Lesuisse C, Wong M, Martin LJ (2011) Epigenetic regulation of motor neuron cell death through DNA methylation. J Neurosci 31:16619–16636
Kirkbride JB, Susser E, Kundakovic M, Kresovich JK, Davey Smith G, Relton CL (2012) Prenatal nutrition, epigenetics and schizophrenia risk: can we test causal effects? Epigenomics 4:303–315
Zhao C, Wang F, Pun FW, Mei L, Ren L, Yu Z, Ng SK, Chen J, Tsang SY, Xue H (2012) Epigenetic regulation on GABRB2 isoforms expression: developmental variations and disruptions in psychotic disorders. Schizophr Res 134:260–266
Lubin FD (2012) Epileptogenesis: can the science of epigenetics give us answers? Epil Curr Am Epil Soc 12:105–110
Zhang K, Schrag M, Crofton A, Trivedi R, Vinters H, Kirsch W (2012) Targeted proteomics for quantification of histone acetylation in Alzheimer’s disease. Proteomics 12:1261–1268
Bihaqi SW, Schumacher A, Maloney B, Lahiri DK, Zawia NH (2012) Do epigenetic pathways initiate late onset Alzheimer disease (LOAD): towards a new paradigm. Curr Alz Res 9:574–588
Diaz de Leon-Guerrero S, Pedraza-Alva G, Perez-Martinez L (2011) In sickness and in health: the role of methyl-CpG binding protein 2 in the central nervous system. Eur J Neurosci 33:1563–1574
Sabunciyan S, Aryee MJ, Irizarry RA, Rongione M, Webster MJ, Kaufman WE, Murakami P, Lessard A, Yolken RH, Feinberg AP, Potash JB (2012) Genome-wide DNA methylation scan in major depressive disorder. PLoS One 7:e34451
Nagy C, Turecki G (2012) Sensitive periods in epigenetics: bringing us closer to complex behavioral phenotypes. Epigenomics 4(4):445–457
Marian AJ (2012) Elements of ‘missing heritability’. Curr Opin Cardiol 27:197–201
Inoue K, Tanabe Y, Lupski JR (1999) Myelin deficiencies in both the central and the peripheral nervous systems associated with a SOX10 mutation. Ann Neurol 46:313–318
Guo JU, Ma DK, Mo H, Ball MP, Jang MH, Bonaguidi MA, Balazer JA, Eaves HL, Xie B, Ford E, Zhang K, Ming GL, Gao Y, Song H (2011) Neuronal activity modifies the DNA methylation landscape in the adult brain. Nat Neurosci 14(10):1345–1351
Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, Agarwal S, Iyer LM, Liu DR, Aravind L, Rao A (2009) Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science 324:930–935
Hohman RJ, Guitton MC, Veron M (1985) Inactivation of S-adenosyl-l-homocysteine hydrolase by cAMP results from dissociation of enzyme-bound NAD+. Proc Natl Acad Sci U S A 82:4578–4581
Bhasin M, Reinherz EL, Reche PA (2006) Recognition and classification of histones using support vector machine. J Comput Biol 13:102–112
Sterner DE, Berger SL (2000) Acetylation of histones and transcription-related factors. Microbiol Mol Biol Rev 64:435–459
Zhang Y, Reinberg D (2001) Transcription regulation by histone methylation: interplay between different covalent modifications of the core histone tails. Genes Dev 15:2343–2360
Klose RJ, Zhang Y (2007) Regulation of histone methylation by demethylimination and demethylation. Nat Rev Mol Cell Biol 8:307–318
Nowak SJ, Corces VG (2004) Phosphorylation of histone H3: a balancing act between chromosome condensation and transcriptional activation. Trends Genet 20:214–220
Shilatifard A (2006) Chromatin modifications by methylation and ubiquitination: implications in the regulation of gene expression. Annu Rev Biochem 75:243–269
Nathan D, Ingvarsdottir K, Sterner DE, Bylebyl GR, Dokmanovic M, Dorsey JA, Whelan KA, Krsmanovic M, Lane WS, Meluh PB, Johnson ES, Berger SL (2006) Histone sumoylation is a negative regulator in Saccharomyces cerevisiae and shows dynamic interplay with positive-acting histone modifications. Genes Dev 20:966–976
Hassa PO, Haenni SS, Elser M, Hottiger MO (2006) Nuclear ADP-ribosylation reactions in mammalian cells: where are we today and where are we going? Microbiol Mol Biol Rev 70:789–829
Cuthbert GL, Daujat S, Snowden AW, Erdjument-Bromage H, Hagiwara T, Yamada M, Schneider R, Gregory PD, Tempst P, Bannister AJ, Kouzarides T (2004) Histone deimination antagonizes arginine methylation. Cell 118:545–553
Wang Y, Wysocka J, Sayegh J, Lee YH, Perlin JR, Leonelli L, Sonbuchner LS, McDonald CH, Cook RG, Dou Y, Roeder RG, Clarke S, Stallcup MR, Allis CD, Coonrod SA (2004) Human PAD4 regulates histone arginine methylation levels via demethylimination. Science 306:279–283
Nelson CJ, Santos-Rosa H, Kouzarides T (2006) Proline isomerization of histone H3 regulates lysine methylation and gene expression. Cell 126:905–916
Zhang Y, Fatima N, Dufau ML (2005) Coordinated changes in DNA methylation and histone modifications regulate silencing/derepression of luteinizing hormone receptor gene transcription. Mol Cell Biol 25:7929–7939
Fuks F (2005) DNA methylation and histone modifications: teaming up to silence genes. Curr Opin Genet Dev 15:490–495
Tamaru H, Selker EU (2001) A histone H3 methyltransferase controls DNA methylation in Neurospora crassa. Nature 414:277–283
Cheng X, Blumenthal RM (2010) Coordinated chromatin control: structural and functional linkage of DNA and histone methylation. Biochemistry 49(14):2999–3008
Gilbert N, Thomson I, Boyle S, Allan J, Ramsahoye B, Bickmore WA (2007) DNA methylation affects nuclear organization, histone modifications, and linker histone binding but not chromatin compaction. J Cell Biol 177:401–411
Ooi SK, Qiu C, Bernstein E, Li K, Jia D, Yang Z, Erdjument-Bromage H, Tempst P, Lin SP, Allis CD, Cheng X, Bestor TH (2007) DNMT3L connects unmethylated lysine 4 of histone H3 to de novo methylation of DNA. Nature 448:714–717
Cedar H, Bergman Y (2009) Linking DNA methylation and histone modification: patterns and paradigms. Nat Rev Genet 10:295–304
El-Osta A (2003) DNMT cooperativity–the developing links between methylation, chromatin structure and cancer. Bioessays 25:1071–1084
Rai K, Jafri IF, Chidester S, James SR, Karpf AR, Cairns BR, Jones DA (2010) Dnmt3 and G9a cooperate for tissue-specific development in zebrafish. J Bio Chem 285(6):4110–4121
Martinowich K, Hattori D, Wu H, Fouse S, He F, Hu Y, Fan G, Sun YE (2003) DNA methylation-related chromatin remodeling in activity-dependent BDNF gene regulation. Science 302:890–893
Espada J, Ballestar E, Fraga MF, Villar-Garea A, Juarranz A, Stockert JC, Robertson KD, Fuks F, Esteller M (2004) Human DNA methyltransferase 1 is required for maintenance of the histone H3 modification pattern. J Biol Chem 279:37175–37184
Kim YI (2004) Folate, colorectal carcinogenesis, and DNA methylation: lessons from animal studies. Environ Mol Mutagen 44:10–25
Ulrich CM, Potter JD (2007) Folate and cancer—timing is everything. JAMA 297:2408–2409
Lee CT, Risom T, Strauss WM (2007) Evolutionary conservation of microRNA regulatory circuits: an examination of microRNA gene complexity and conserved microRNA-target interactions through metazoan phylogeny. DNA and cell biology 26:209–218
Iorio MV, Piovan C, Croce CM (2010) Interplay between microRNAs and the epigenetic machinery: an intricate network. Biochim Biophys Acta 1799:694–701
Huang J, Wang Y, Guo Y, Sun S (2010) Down-regulated microRNA-152 induces aberrant DNA methylation in hepatitis B virus-related hepatocellular carcinoma by targeting DNA methyltransferase 1. Hepatology 52:60–70
Braconi C, Huang N, Patel T (2010) MicroRNA-dependent regulation of DNA methyltransferase-1 and tumor suppressor gene expression by interleukin-6 in human malignant cholangiocytes. Hepatology 51:881–890
Han M, Toli J, Abdellatif M (2011) MicroRNAs in the cardiovascular system. Curr Opin Cardiol 26:181–189
Bak M, Silahtaroglu A, Moller M, Christensen M, Rath MF, Skryabin B, Tommerup N, Kauppinen S (2008) MicroRNA expression in the adult mouse central nervous system. RNA 14:432–444
Smalheiser NR, Lugli G (2009) microRNA regulation of synaptic plasticity. Neuromol Med 11:133–140
Mattson MP (2003) Methylation and acetylation in nervous system development and neurodegenerative disorders. Ageing Res Rev 2:329–342
Namihira M, Nakashima K, Taga T (2004) Developmental stage dependent regulation of DNA methylation and chromatin modification in a immature astrocyte specific gene promoter. FEBS Lett 572:184–188
Persengiev SP, Kilpatrick DL (1996) Nerve growth factor induced differentiation of neuronal cells requires gene methylation. Neuroreport 8:227–231
Frey L, Hauser WA (2003) Epidemiology of neural tube defects. Epilepsia 44(Suppl 3):4–13
Doolin MT, Barbaux S, McDonnell M, Hoess K, Whitehead AS, Mitchell LE (2002) Maternal genetic effects, exerted by genes involved in homocysteine remethylation, influence the risk of spina bifida. Am J Hum Gen 71:1222–1226
Copp AJ, Greene ND (2010) Genetics and development of neural tube defects. J Pathol 220:217–230
Shookhoff JM, Gallicano GI (2010) A new perspective on neural tube defects: folic acid and microRNA misexpression. Genesis 48:282–294
Suren P, Roth C, Bresnahan M, Haugen M, Hornig M, Hirtz D, Lie KK, Lipkin WI, Magnus P, Reichborn-Kjennerud T, Schjolberg S, Davey Smith G, Oyen AS, Susser E, Stoltenberg C (2013) Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. JAMA 309:570–577
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Meethal, S.V., Hogan, K.J., Mayanil, C.S. et al. Folate and epigenetic mechanisms in neural tube development and defects. Childs Nerv Syst 29, 1427–1433 (2013). https://doi.org/10.1007/s00381-013-2162-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-013-2162-0