Abstract
Earthworms (Annelida: Oligochaeta) deposit several tons per hectare of casts enriched in nutrients and/or arbuscular mycorrhizal fungi (AMF) and create a spatial and temporal soil heterogeneity that can play a role in structuring plant communities. However, while we begin to understand the role of surface casts, it is still unclear to what extent plants utilize subsurface casts. We conducted a greenhouse experiment using large mesocosms (volume 45 l) to test whether (1) soil microsites consisting of earthworm casts with or without AMF (four Glomus taxa) affect the biomass production of 11 grassland plant species comprising the three functional groups grasses, forbs, and legumes, (2) different ecological groups of earthworms (soil dwellers—Aporrectodea caliginosa vs. vertical burrowers—Lumbricus terrestris) alter potential influences of soil microsites (i.e., four earthworms × two subsurface microsites × two AMF treatments). Soil microsites were artificially inserted in a 25-cm depth, and afterwards, plant species were sown in a regular pattern; the experiment ran for 6 months. Our results show that minute amounts of subsurface casts (0.89 g kg−1 soil) decreased the shoot and root production of forbs and legumes, but not that of grasses. The presence of earthworms reduced root biomass of grasses only. Our data also suggest that subsurface casts provide microsites from which root AMF colonization can start. Ecological groups of earthworms did not differ in their effects on plant production or AMF distribution. Taken together, these findings suggest that subsurface earthworm casts might play a role in structuring plant communities by specifically affecting the growth of certain functional groups of plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In many temperate grassland soils, earthworms play key roles in linking below- and aboveground processes in ecosystems including plant growth (Lavelle 1988; Scheu 2003; Bardgett and Wardle 2010). Within 1 year, earthworms can egest up to 250 tons ha−1 of nutrient-rich casts below and on the surface of soils (Bohlen 2002). On the soil surface, casts are rather heterogeneously distributed showing associations with certain plant species and thereby specifically stimulating their growth (Zaller and Arnone 1999). Thus, earthworm casting activity leads to spatially and temporally heterogeneous soil resources which can be specifically utilized by plant species (Jackson and Caldwell 1992; Bilbrough and Caldwell 1997; Farley and Fitter 1999), eventually affecting the structure of plant communities. However, while we have some understanding on interactions between earthworm surface casts and plant species (Springett and Syers 1979; Zaller and Arnone 1999; Decaens et al. 2003; Zaller and Saxler 2007), we know virtually nothing regarding the functional significance of subsurface casts for plant species assemblages.
In temperate grasslands, three ecological groups of earthworms are distinguished: epigeic species which live in the top soil layer building no permanent burrows, anecic species drilling vertical burrows reaching from the soil surface down to the mineral soil layer, and endogeic species burrowing in the upper mineral soil layer (Bouché 1977). When studying anecic (Lumbricus spp.) and endogeic species (Aporrectodea spp.) in laboratory, it has been shown that, depending on temperature, up to 90 % of earthworm casts are deposited at the soil surface (Whalen et al. 2004). Besides temperature, soil bulk density also affects cast production on the soil surface (Joschko et al. 1989). These earthworm casts not only contain much more organic C, nutrients, and microorganisms than the surrounding soil (Zhang and Schrader 1993; Zaller and Arnone 1997; Buck et al. 1999; Haynes et al. 2003; Aira et al. 2005) but they can also contain considerably higher numbers of arbuscular mycorrhizal fungi (AMF) spores and propagules than undigested field soil (Reddell and Spain 1991; Gange 1993). Indeed, earthworms have been shown to feed on mycorrhizal structures (Bonkowski et al. 2000) and to affect mycorrhizal colonization of plant roots (Yu et al. 2005; Zarea et al. 2009; Ll et al. 2012). While mycorrhizal fungi enhance the phosphorus and nitrogen uptake of plants, the fungi are also dependent on plants as a host for their carbon nutrition (Smith and Read 2008). Therefore, plant species can differ in their degree of benefit they receive from different mycorrhizal symbionts (van der Heijden et al. 1998; Klironomos 2003; Zaller et al. 2011a). The few studies investigating interactions between earthworms and AMF on plant productivity have suggested that interactions between earthworms and AMF are affecting plant growth in various directions (Milleret et al. 2009; Zarea et al. 2009; Zaller et al. 2011a, b) or are of minor importance (Wurst et al. 2004; Eisenhauer et al. 2009).
In order to investigate the role of subsurface casts of earthworms for grassland plant species, we conducted a full-factorial greenhouse experiment where artificial soil microsites, consisting of earthworm casts with or without AMF, were established. The experimental setup comprised 11 grassland plant species (grasses, forbs, and legumes), 2 earthworm species comprising anecic and endogeic ecotypes, and 4 AMF species of the taxon Glomus. We hypothesized that (1) different functional groups of grassland plants (i.e., grasses, forbs, legumes) differ in their ability to utilize subsurface earthworm casts, (2) different ecological groups of earthworms (i.e., anecics vs. endogeics) due to their different feeding and burrowing habits have different effects on AMF distribution and plant growth, and (3) AM fungi present in soil microsites will be more efficiently distributed among plants when earthworms are present.
Materials and methods
Experimental setup and treatments
We conducted the experiment using plastic pots (diameter 45 cm, height 35 cm, volume 45 l; further called mesocosms) in a greenhouse of the University of Natural Resources and Life Sciences, Vienna, Austria from August 2008 until March 2009. The experiment was conducted under ambient light, and the mean air temperature during the course of the experiment was 17.8 ± 2.4 °C at 65 ± 5 % relative humidity. Mesocosms were filled with 40 l of a 1:2 v/v mixture of field soil (Haplic Chernozem, silty loam) and quartz sand (mean grain size 1.4–2.2 mm). The characteristics of this mixture were as follows: pH, 7.6; C org, 2.2 %; N tot, 0.102 ± 0.003 %; total K, 119.3 ± 0.9 mg kg−1; total P, 62.33 ± 0.33 mg kg−1; and bulk density 1.6 g cm−3 (Zaller et al. 2011b). Field soil was obtained from an arable field at the University’s Research Farm Groß-Enzersdorf, sieved (mesh size 1 cm), mixed with fire-sterilized quartz sand, and steam-sterilized (110 °C for 3 h). This soil–quartz sand mixture has been successfully used in previous experiments including the same plant, earthworm, and AMF taxa (Heiner et al. 2011; Putz et al. 2011; Zaller et al. 2011c).
The three-factorial experimental design consisted of the factor earthworms (four levels: addition of only endogeic worms, Apo; addition of only anecic worms, Lum; addition of a mix of endogeic and anecic worms, ApoLum; no earthworm addition, −EW; see below for more details), subsurface microsite (two levels: microsite consisting of earthworm casts, +cast; microsite consisting of soil, −cast), and AMF inoculation (two levels: inoculation of microsites with a mix of four active Glomus taxa, +AMF; inoculation of subsurface microsites with a mix of four sterilized Glomus taxa, −AMF; Fig. 1). More details on the taxa used can be found below. Each treatment was replicated six times making up a total of 96 mesocosms (four earthworms × two subsurface microsites × two AMF treatments × six replicates).
The experimental setup to test the effects of earthworms (Apo—endogeic Aporrectodea caliginosa, Lum—anecic Lumbricus terrestris, ApoLum—mixture of Aporrectodea caliginosa and Lumbricus terrestris, −EW—no earthworms added) on the grassland plant utilization of soil microsites with/without earthworm castings (−casts/+casts) and/or arbuscular mycorrhizal fungi inoculum (−AMF/+AMF). Modified after
To prevent earthworms from escaping, the drainage holes of the pots were covered with water-permeable fleece material. Additionally, a 20-cm-high barrier of transparent plastic coated with soft soap on the upper 2 cm was attached to the upper rim of the pots. Fleece and barriers were also installed on pots containing no earthworms to ensure similar microclimatic conditions between different treatments.
Earthworm inoculation
We used the anecic Lumbricus terrestris L. and the endogeic Aporrectodea caliginosa (Savigny 1826) species; both species are common in temperate grasslands throughout Europe. Treatment Apo received 10 individuals (in total 5.7 ± 0.1 g fresh weight) of adult/subadult Aporrectodea caliginosa, treatment Lum received 2 individuals (in total 8.3 ± 0.2 g fresh weight (fwt)) of adult Lumbricus terrestris, and treatment ApoLum received 5 individuals of Aporrectodea caliginosa and 1 individual of Lumbricus terrestris (in total 3 ± 0.1 g fwt plus 4.4 ± 0.2 g fwt for Apo + Lum). Aporrectodea caliginosa was collected by hand digging from an agricultural field (Landwirtschaftliche Bundesversuchswirtschaften Königshof, Wilfleinsdorf, Austria), while Lumbricus terrestris was obtained from a commercial supplier (Denuwurm, Stuttgart, Germany). All earthworms were kept in sterilized soil in a climate chamber (15 °C) for 2 weeks, carefully rinsed under tap water, and inserted to the mesocosms.
Planting
Each mesocosm was planted with 11 low-fertile grassland species, representing the three major groups grasses (Arrhenatherum elatius L., Bromus erectus Huds., Dactylis glomerata L.), forbs (Hieracium pilosella L., Knautia arvensis (L.) Coult., Leucanthemum vulgare Lam., Plantago lanceolata L., Prunella vulgaris L., Salvia pratensis L.), and legumes (Lotus corniculatus L., Trifolium pratense L.). Seeds were obtained from a commercial supplier (Rieger-Hofmann GmbH, Blaufelden, Germany) and germinated on a wet filter paper before transplanted into the mesocosms in a regular pattern along two concentric circles. All plant species used are common in temperate grasslands throughout Europe. The outer circle had a diameter of about 32 cm and contained two individuals of each plant species, with the exception of Arrhenatherum elatius which was present with four individuals on two spots with two individuals each. The inner circle had a diameter of about 16 cm and contained one individual of each species with the exception of Arrhenatherum elatius which was present two times. In total, 24 plants were planted in each mesocosm. Dead plants were consistently replaced by new seedlings during the first weeks of the experiment. The mesocosms were watered daily with a constant amount of tap water for all treatments. The positions of mesocosms were completely randomized initially every 3 weeks to account for possible environmental gradients within the glasshouse.
Installation of soil microsites
One week after planting, we inserted one microsite at a 25-cm soil depth in the middle of each mesocosm using a 5-cm diameter corer (Fig. 1). The microsite consisted of a plastic grid pot (diameter 5 cm, height 7 cm, mesh size about 1 cm) commonly used for aquarium plants. Using these grid pots helped to create compact microsites, while enabling earthworms and roots to enter and facilitating the location of the microsite during the final harvest of the mesocosms. According to the specific treatments, these microsites contained either 50 g of casts produced by earthworms of the respective treatment (i.e., for Apo treatment, casts were produced by Aporrectodea caliginosa; for Lum treatment, by Lumbricus terrestris; for ApoLum treatments, 25 g of casts produced by Aporrectodea caliginosa and 25 g produced by Lumbricus terrestris were mixed). Therefore, 10 individuals of Aporrectodea caliginosa or 2 individuals of Lumbricus terrestris were separately held in plastic boxes containing 500 g field soil and regularly fed with ground oatmeal in order to produce casts; five replicates of this setting were prepared. After 2 weeks, all soil in the boxes was readily ingested and excreted again so that all material in the boxes consisted of casts. This material was then filled into the grid pots and inserted into the mesocosms. The microsite structures created here are similar in size to those that can be found along a grassland soil profile with normal earthworm activity.
In −cast treatments, these microsites contained only sterilized field soil. In +AMF treatments, microsites additionally contained 25 g of AMF inoculum. The same amount of sterilized inoculum was added to the −AMF treatments. The inoculum consisted of a mixture of clay granules, infected root pieces, and AM fungi spores of Glomus claroideum, Glomus intraradices, Glomus mossae, and Glomus geosporum (Symbio-m, Lanškroun, Czech Republic). Each mesocosm also received 10 ml of a microbial wash prepared by wet sieving 1,000 g inoculum and 3,000 g of field soil through a series of sieves (finest sieve was 10 μm which holds back mycorrhizal structures) into a final volume of 960 ml to correct for possible differences in microbial communities other than mycorrhiza between field soil and sterilized soil mixture (Koide and Li 1989).
Prior to insertion into the mesocosms, we took three subsamples of each microsite treatment and analyzed their inorganic ammonium-N and nitrate-N concentration in 0.5 M K2SO4 extracts (1:5 w/v) using the modified indophenol blue technique (Sims et al. 1995), with a Bio-Rad Microplate Reader 550 (Table 1).
Measurements
Earthworm surface casts were collected once a month, dried at 40 °C for 24 h, and weighed; cumulative surface cast production was used for statistical analysis. Once a week, we surveyed the mesocosms for dead earthworms lying on the soil surface. Dead earthworms were only found during the first couple of weeks and were replaced immediately with new specimens living in sterile soil. We also regularly sampled plant roots from each mesocosm using a 1-cm soil corer to check for the presence of AMF colonization after staining with vinegar and ink (Vierheilig et al. 1998); soil voids were filled back with original soil after sorting out the roots.
Because the rapidly growing grasses Arrhenatherum elatius and D. glomerata would overgrow all other plants in the mesocosms, we cut them at 2 cm above soil surface 13 weeks after starting the experiment. Mesocosms were harvested destructively 6 months after the start of the experiment by flipping over the pots and carefully taking out the individual plants. All mesocosms contained the same plant density (e.g., 24 plant individuals). We only used Arrhenatherum elatius and D. glomerata data from the second harvest in the further analyses. Maximum shoot lengths were measured before aboveground parts were dried at 40 °C to determine their dry mass. Roots were cut off and stored at 4 °C until they were washed free of soil using tap water, dried at 40 °C, and weighed. From these roots, we randomly selected subsamples from three replicates of each treatment from T. pratense, Arrhenatherum elatius, S. pratensis, B. erectus, Plantago lanceolata, and Leucanthemum vulgare for AMF DNA analysis (see below). Plant roots which grew into the microsites were sampled from the same mesocosms. Roots growing into microsites were sorted out, dried at 50 °C for 24 h, and weighed. Root parts that could not be assigned to a species were collected and considered as bulk roots in order to determine total mesocosm root production. At harvest, all live and dead earthworms were counted; only the live earthworms were weighed.
Analysis of AMF DNA in plant roots and aboveground earthworm casts
Root and cast samples were oven-dried (60 °C) and milled to a fine powder in a beat beater (FastPrep120, Bio101) with Lysing Matrix A (MP Biomedicals GmbH). DNA was extracted and purified from the powder by LGC Genomics (Germany). A 1:20 dilution of the DNA was used as a template. For sensitive detection of the four Glomus species from the inoculum in root and cast samples, a nested PCR was performed. The first amplification was carried out with two Glomus-specific forward primers (GlGrA and GlGrB, Schüßler et al. 2001) and a universal reverse primer (SSU-1536-3, Borneman and Hartin 2000). The second amplification step made use of the primer pair AM1/NS31 (Simon et al. 1992; Helgason et al. 1998). The PCR conditions were as follows: 10 μl 2× GoTaq Green Master Mix (Promega), 10 μg BSA, 20 pmol forward primer, 20 pmol reverse primer, 0.5 μl template DNA, and 20 μl ultrapure water. Cycling parameters for the first PCR were as follows: initial denaturation at 95 °C for 2 min and 30 s followed by 30 cycles of denaturation at 94 °C for 30 s, annealing at 57 °C for 30 s and extension at 72 °C for 1 s, and a final extension at 72 °C for 5 min. For the nested PCR, the following cycling parameters were changed: the annealing temperature was raised to 60 °C and the extension time was shortened to 30 s. PCR products were separated on an agarose gel and visualized under UV. Quality control of sample DNA was performed with the fungal-specific primer pair ITS1F/ITS4 (White et al. 1990; Gardes and Bruns 1993) and cycling parameters as above except that the annealing temperature was set to 54 °C, extension time to 45 s, and cycle number to 35. From all root and cast samples, fungal DNA could be amplified.
Statistical analysis
For all statistical analyses, the normal distribution of the data was tested by the Kolmogorov–Smirnov test, homogeneity of variances by the Levene’s test. If necessary, data were transformed by natural logarithm to improve normality and homogenize the variances (Köhler et al. 2002). The biomass data (root and shoot dry weight) of the plant individuals were averaged per species and mesocosm in order to prevent pseudoreplication. For generating the biomass data per plant group (grasses, forbs, legumes), the values of the respective species were summed up. Effects of the factors “Casts” (±earthworm casts in the microsites), “AMF” (±AMF inoculate in the microsites), and “Earthworms” (±three different earthworm population treatments in the mesocosms) on plant biomass and earthworm performance (fresh weight and cast activity) were analyzed by three-factorial ANOVAs. In case of the factor “Earthworms,” Tukey’s post hoc tests were conducted. When analyzing the earthworm data, only the mesocosms containing earthworms, but not the −EW, were included. Casting activity during the course of the experiment was analyzed using repeated measures ANOVAs with five sampling dates and earthworm treatments as factors (−EW not included). Relationships between plant biomass data and earthworm activity were described and tested by Pearson correlation coefficients. Effects of the factors “Casts” and “Earthworms” on the measured mycorrhization rates inside and outside the microsites were analyzed by chi-squared tests.
All statistical analyses were performed with SPSS 11.5.1 (SPSS Inc., Chicago, IL, USA). Data of plant biomass, earthworm fresh mass, and earthworm activity are given as means per mesocosm ± standard error (SE) of the mean.
Results
Root and shoot biomass of both forbs and legumes were significantly lower in mesocosms containing soil microsites with casts (+cast) than without (−cast; Fig. 2b, c; Table 2). Contrary to that, neither root biomass nor shoot biomass of grasses was significantly affected by cast treatments (Table 2, Fig. 2a). Soil microsites were utilized by plant roots as indicated by roots growing into the microsites; however, there was no difference among the treatments in root biomass present within the soil microsites (root biomass in microsites across treatments 27.5 ± 0.6 mg dwt). Root mass of grasses was significantly reduced in earthworm treatments (Table 2, Fig. 2a). AMF in soil microsites significantly decreased total grass shoot biomass due to affecting Arrhenatherum elatius (Table 2, Fig. 2a).
Shoot and root dry mass of plant functional groups in response to earthworms and soil microsites with arbuscular mycorrhizal fungi and/or earthworm casts. Means ± SE, n = 6. For the abbreviations, see Fig. 1
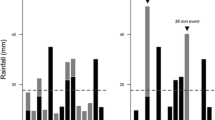
Aboveground casting activity was consistently high throughout the entire experiment but did not differ significantly between earthworm treatments (repeated measures ANOVA; Fig. 3a, b). There was a trend towards increased casting activity of Aporrectodea caliginosa and decreased activity of Lumbricus terrestris in the last month of the experiment (Fig. 3a, b). Neither the number nor the mass of surface cast production was affected by earthworm treatments or the presence of AMF in subsurface microsites. However, both number and mass of surface casts were significantly reduced by the presence of casts in soil microsites (Table 3). At the destructive harvest, we generally found less earthworm biomass than initially inserted (Fig. 3c). Percent change from initially added earthworm numbers was 52, 88, and 71 % for Apo, Lum, and ApoLum, respectively (difference between initially added numbers and end P < 0.05); included here are 2.3 ± 0.6, 0.5 ± 0.1, and 1.1 ± 0.3 dead earthworms per treatment found during harvest for Apo, Lum, and ApoLum, respectively. Aboveground cast production in Lum and ApoLum treatments was significantly and positively correlated with forb root mass (Fig. 4).
Earthworm activity during the course of the experiment (means ± SE, n = 24) and percent change in earthworm fresh mass at harvest from initial fresh mass in response to earthworm treatments and soil microsites containing inoculum of arbuscular mycorrhizal fungi and earthworm casts (means ± SE, n = 6). For the abbreviations, see Fig. 1
There was a significant interaction between earthworm and AMF treatments regarding their effects on forb roots (Table 2). Within ApoLum treatments, mycorrhizal fungi in soil microsites decreased forb root biomass (Fig. 5). In contrast, AM fungi in soil microsites did not affect forb root biomass in the other treatments (Fig. 5). Aboveground biomass production of grasses, particularly of Arrhenatherum elatius, was significantly lower when subsurface microsites contained AMF (Table 2). No AMF could be detected in roots by the staining technique, which indicates very low colonization rates (data not shown).
Forbs root dry weight in response to earthworm treatments and presence of arbuscular mycorrhizal fungi in soil microsites. Means ± SE, n = 6. The asterisk denotes significant difference (P < 0.05), while ns denotes no significant difference (P > 0.05). For the abbreviations, see Fig. 1
The rate of AMF detection in roots was low: out of 192 root subsamples taken and tested on mycorrhization using PCR, only 16 samples (8.3 %) were tested positive (Fig. 6). AMF detection rates were not affected by earthworms or cast presence in microsites (AMF in roots from microsites: Cast: P = 0.317, EW: P = 0.766; AMF in roots from outside of microsites: Cast: P = 0.196, EW: P = 0.430). However, there was a pattern towards more frequent AMF detection in treatments where no casts were present in soil microsites. This trend was not recognizable within subsamples taken from microsites (Fig. 6).
Total AMF detection using PCR of selected plant species (T. pratense, Arrhenatherum elatius, S. pratensis, B. erectus, Plantago lanceolata, and Leucanthemum vulgare) in response to earthworm treatments and soil microsites containing inoculum of arbuscular mycorrhizal fungi and earthworm casts within microsites (a) and mesocosms (b). Missing columns mean that no AMF was detected. The resulting P values of the chi-squared test: a Cast: P = 0.317, EW: P = 0.766; b Cast: P = 0.196, EW: P = 0.430. For the abbreviations, see Fig. 1
Discussion
This study for the first time shows that subsurface earthworm casts are important soil microsites that can specifically influence forbs and legumes (but not grasses) in model grasslands. This is especially interesting as these effects were caused by only 50 g of earthworm casts located in a 25-cm depth within 56,000 g of soil in the experimental mesocosms. Effects on plants were little influenced by different ecological groups of earthworms. Results also suggest that minute amounts of AMF within these subsurface (0.44 kg−1) microsites can colonize plant roots.
Subsurface casts and plant production
Shoot and root biomass of forbs and legumes were found to be lower in +cast treatments, while grass biomass production remained unaffected. The lack of response to casts of grasses is somewhat surprising as earlier studies showed a positive correlation between surface casts and grasses (Tomati et al. 1988; Zaller and Arnone 1999). Despite the unresponsiveness to additional nutrients (i.e., ammonium-N, nitrate-N; Table 1) provided by subsurface casts, grasses were the plant group with the highest biomass production above- and belowground (see also Eisenhauer and Scheu 2008). We conclude that grasses, due to their large and fast-growing root systems, exploited the available soil volume faster than forbs or legumes being less responsive to nutrient-rich microsites (Farley and Fitter 1999; Zaller 2007). Thus, grasses may have prevented highest possible biomass production of forbs or legumes (Hooper 1998). Another reason for the non-response of grasses might be that in our systems, nutrients were not limiting plant nutrition and thus casts did not affect grass production.
The presence of roots within microsites showed that nutrients of these spots were indeed utilized by plants. However, the biomass of roots which grew into the microsites did not differ between cast treatments despite the fact that mineral-N concentrations in microsites with casts were considerably higher than in microsites containing soil only (Table 1). It might well be that more fine roots grew into cast microsites but could not be detected by measuring root biomass only. We and others (Springett and Syers 1979; Spiers et al. 1986; Zaller and Arnone 1999) have observed root proliferation into casts, indicating that plants can exploit these nutrients. Differences in the abilities of species to exploit nutrient patches may alter the competitive balance among plant species in plant communities (Bilbrough and Caldwell 1995, Tibbett 2000). The finding that the presence of subsurface casts significantly decreased earthworm surface cast production both in cast numbers and mass can be explained by several direct and indirect effects. First, aside from plant roots, microorganisms associated with subsurface casts might be an additional food source for earthworms (Curry and Schmidt 2007). Particularly the endogeic earthworm Aporrectodea caliginosa utilizes subterraneous food sources and therefore deposits casts less frequently on the soil surface. Second, subsurface casts decreased root mass production and a significant positive correlation between forb root mass and surface cast production indicates that roots stimulated earthworm activity (Shipitalo et al. 1988; Zaller and Arnone 1997). Vice versa, root herbivory by earthworms could also stimulate root production (Cortez and Bouché 1992; Gange and Brown 2002). We found some indication for this as in the three earthworm treatments (Apo, Lum, and ApoLum), at least the root biomass of the grasses was significantly lower than in the treatments without earthworms. The reason for that might be a negative effect of earthworms on plant root biomass by their feeding behavior; however, this remains to be investigated in more detail. In our experiment, the chemical quality of casts produced from different ecological groups of earthworms was not different. We explain this mainly by the fact that all earthworms were fed with oatmeal while under natural conditions, the different feeding behavior might have resulted in different cast qualities (Curry and Schmidt 2007).
We observed a marked decline of earthworm biomass during the course of the experiment in all earthworm treatments. Such declines are frequently observed in earthworm laboratory studies, especially when experiments lasted several months (Wurst et al. 2004; Zaller et al. 2011c). For the current experiment, the low earthworm recovery might arise from the initially quite low biomasses of individual earthworms especially of Aporrectodea caliginosa and/or the pretreatments in sterile soil (Fründ et al. 2010). However, based on the fact that earthworm casting activity changed little until the end of the experiment, we assume that (1) the decline of earthworm individuals and their loss of weight might have occurred within the last week before harvest when we stopped watering to facilitate harvesting of root systems of individual plant species and (2) some earthworms probably also naturally died during the course of the experiment or might have escaped despite above- and belowground barriers.
Effect of AMF in subsurface microsites
Overall, the rate of AMF detection using PCR was low in our experiment. We attribute this mainly to the fact that only a minute amount of 0.44 g kg−1 AMF inoculum was added on a single spot to the soil mass in the mesocosms. Knowing that the presence of soil microorganisms such as Azotobacter and Pseudomonas can produce growth substances that can increase the mycorrhizal colonization of plants (Smith and Read 2008), we amended microbial wash to all mesocosms. Nevertheless, given this small amount of inoculum within the mesocosms, it is remarkable that AM fungi significantly affected (reduced) the shoot biomass of the grasses.
In our study, subsurface casts did not increase AMF detection rate in roots within microsites. Subsurface casts however reduced AMF in roots outside the microsites, probably due to the increased availability of nutrients as the outcomes of several studies indicated that AMF root colonization was reduced when N and P were available in sufficient concentrations for the plants (Abbott et al. 1984; Liu et al. 2000). To what extent earthworms acted as vectors for AMF spores remains to be investigated. The finding that AMF-infected roots outside the microsites were also found in mesocosms without earthworms restricted to the −cast treatment suggests that the enhanced earthworm activity within the −cast treatments can only partly explain the positive effect on the measured mycorrhization rate.
The only significant interaction between earthworms and AMF was seen on forb roots when anecic and endogeic earthworms were active. Whereas forb root biomass was reduced by the presence of AM fungi and earthworms, this effect was not observed within treatments without earthworms (−EW) or in treatments which just contained anecic Lumbricus terrestris (Lum, Fig. 4). Others also observed a reduction of plant biomass in systems which contained both earthworms and AMF because earthworms reduced the positive effect of AMF on root biomass within the symbiosis (Milleret et al. 2009; Zaller et al. 2011b, c). Interactions between earthworms and AMF on plant production might depend on the behavior of the respective earthworm species. It is possible that mycorrhized plant roots have a similar effect on the earthworm feeding behavior (Bonkowski et al. 2000). In our case, we suggest that particularly Aporrectodea caliginosa reduced root biomass of forbs within AMF treatments as a result of its preference for mycorrhized forb roots. This suggestion is based on the higher mycorrhization rate in treatments containing Aporrectodea caliginosa. However, experimental design of the current study does not allow to state whether the endogeic Aporrectodea caliginosa might have more effects on the distribution of mycorrhizal fungi than the anecic Lumbricus terrestris.
Conclusions
Taken collectively, our results demonstrate that (1) different functional groups of grassland plants differ in their ability to utilize subsurface earthworm casts, (2) different earthworm functional groups seemed to have similar (few) effects on plant biomass production showing little interaction with subsurface casts, and (3) there is some indication that AM fungi located in subsurface casts are utilized by different plant species; however, earthworms only seem to play a minor role in distributing AMF among plants. While the present results corroborate other studies on interactions between surface casts and plant species (Zaller and Arnone 1999; Arnone et al. 2013), more in-depth investigations including the nutrient contents of plants, using field collected earthworm casts and perhaps a less complex setting with only one earthworm species, would help to better understand the underlying mechanisms. A challenge for the future will also be to understand the roles of both surface and subsurface earthworm casts in affecting plant communities in the field. Potential tools for tracking these interactions using stable isotope tracers have recently been introduced (Heiner et al. 2011; Putz et al. 2011).
References
Abbott L, Robson A, De Boer G (1984) The effect of phosphorus on the formation of hyphae in soil by the vesicular-arbuscular mycorrhizal fungus, Glomus fasciculatum. New Phytologist 97:437–446
Aira M, Monroy F, Domínguez J (2005) Ageing effects on nitrogen dynamics and enzyme activities in casts of Aporrectodea caliginosa (Lumbricidae). Pedobiol 49:467–473
Arnone JA, Zaller JG, Hofer G, Schmid B, Körner C (2013) Loss of plant biodiversity eliminates stimulatory effect of elevated CO2 on earthworm casting activity in grasslands. Oecologia 171:613–622
Bardgett RD, Wardle DA (2010) Aboveground-belowground linkages. Biotic interactions, ecosystem processes, and global change. Oxford series in ecology and evolution. Oxford University Press, Oxford
Bilbrough CJ, Caldwell MM (1995) The effects of shading and N status on root proliferation in nutrient patches by the perennial grass Agropyron desertorum in the field. Oecologia 103:10–16
Bilbrough CJ, Caldwell MM (1997) Exploitation of springtime ephemeral N pulses by six Great Basin plant species. Ecology 78:231–243
Bohlen PJ (2002) Earthworms. In: Lal R (ed) Encyclopedia of soil science. Marcel Dekker, New York, pp 370–373
Bonkowski M, Griffiths BS, Ritz K (2000) Food preferences of earthworms for soil fungi. Pedobiol 44:666–676
Borneman J, Hartin R (2000) PCR primers thar amplify fungal rRNA genes from environmental samples. Appl Environ Microbiol 66:4356–4366
Bouché MB (1977) Strategies lombriciennes. In: Lohm U, Persson T (eds) Soil organisms as components of ecosystems, vol 25. Ecological Bulletins, Stockholm, pp 122–133
Buck C, Langmaack M, Schrader S (1999) Nutrient content of earthworm casts influenced by different mulch types. Europ J Soil Biol 35:23–30
Cortez J, Bouché MB (1992) Do earthworms eat living roots? Soil Biol Biochem 24:913–915
Curry JP, Schmidt O (2007) The feeding ecology of earthworms—a review. Pedobiol 50:463
Decaens T, Mariani L, Betancourt N, Jimenez JJ (2003) Seed dispersion by surface casting activities of earthworms in Colombian grasslands. Acta Oecologica 24:175–185
Eisenhauer N, Milcu A, Sabais ACW, Bessler H, Weigelt A, Engels C, Scheu S (2009) Plant community impacts on the structure of earthworm communities depend on season and change with time. Soil Biol Biochem 41:2430–2443
Eisenhauer N, Scheu S (2008) Earthworms as drivers of the competition between grasses and legumes. Soil Biol Biochem 40:2650–2659
Farley RA, Fitter AH (1999) The response of seven co-occurring woodland herbaceous perennials to localized nutrient-rich patches. J Ecol 87:849–859
Fründ H-C, Butt K, Capowiez Y, Eisenhauer N, Emmerling C, Ernst G, Potthoff M, Schädler M, Schrader S (2010) Using earthworms as model organisms in the laboratory: recommendations for experimental implementation. Pedobiol 53:119–125
Gange AC (1993) Translocation of mycorrhizal fungi by earthworms during early succession. Soil Biol Biochem 25:1021–1026
Gange AC, Brown VK (2002) Soil food web components affect plant community structure during early succession. Ecol Res 17:217–227
Gardes M, Bruns T (1993) ITS primers with enhanced specificity for basidiomycetes—application to the identification of mycorrhizae and rusts. Molec Ecol 2:113–118
Haynes RJ, Fraser PM, Piercy JE, Tregurtha RJ (2003) Casts of Aporrectodea caliginosa (Savigny) and Lumbricus rubellus (Hoffmeister) differ in microbial activity, nutrient availability and aggregate stability. Pedobiol 47:882–887
Heiner B, Drapela T, Frank T, Zaller JG (2011) Stable isotope 15N and 13C labelling of different functional groups of earthworms and their casts: a tool for studying trophic links. Pedobiol 54:169–175
Helgason T, Daniell TJ, Husband R, Fitter AH, Young JPY (1998) Ploughing up the wood-wide web? Nature 394:431
Hooper DU (1998) The role of complementarity and competition in ecosystem responses to variation in plant diversity. Ecology 79:704–719
Jackson RB, Caldwell MM (1992) Shading and the capture of localized soil nutrients: nutrient contents, carbohydrates, and root uptake kinetics of a perennial tussock grass. Oecologia 91:457–462
Joschko M, Diestel H, Larink O (1989) Assessment of earthworm burrowing efficiency in compacted soil with a combination of morphological and soil physical measurements. Biol Fertil Soils 8:191–196
Klironomos JN (2003) Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecology 84:2292–2301
Köhler W, Schachtel G, Voleske P (2002) Biostatistik, 3rd edn. Springer, Heidelberg
Koide RT, Li M (1989) Appropriate controls for vesicular-arbuscular mycorrhiza research. New Phytol 111:35–44
Lavelle P (1988) Earthworm activities and the soil system. Biol Fertil Soils 6:237–251
Liu A, Hamel C, Hamilton R, Ma B, Smith D (2000) Acquisition of Cu, Zn, and Fe by mycorrhizal maize (Zea mays L.) grown in soil at different P and micronutrient levels. Mycorrhiza 9:331–336
Ll H, Li X, Dou Z, Zhang J, Wang C (2012) Earthworm (Aporrectodea trapezoides)–mycorrhiza (Glomus intraradices) interaction and nitrogen and phosphorus uptake by maize. Biol Fertil Soils 48:75–85
Milleret R, Le Bayon RC, Gobat JM (2009) Root, mycorrhiza and earthworm interactions: their effects on soil structuring processes, plant and soil nutrient concentration and plant biomass. Plant Soil 316:1–12
Putz B, Drapela T, Wanek W, Schmidt O, Frank T, Zaller JG (2011) A simple method for in-situ-labelling with 15N and 13C of grassland plant species by foliar brushing. Methods Ecol Evol 2:326–332
Reddell P, Spain AV (1991) Earthworms as vectors of viable propagules of mycorrhizal fungi. Soil Biol Biochem 23:767–774
Scheu S (2003) Effects of earthworms on plant growth: patterns and perspectives. Pedobiol 47:846–856
Schüßler A, Gehrig H, Schwarzott D, Walker C (2001) Analysis of partial Glomales SSU rRNA gene sequences: implications for primer design and phylogeny. Mycol Res 105:5–15
Shipitalo MJ, Protz R, Tomlin AD (1988) Effect of diet on the feeding and casting activity of Lumbricus terrestris and L. rubellus in laboratory culture. Soil Biol Biochem 2:233–237
Simon L, Lalonde M, Bruns T (1992) Specific amplification of 18S fungal ribosomal genes from vesicular-arbuscular endomycorrhizal fungi colonizing roots. Appl Environ Microbiol 58:291–295
Sims GK, Ellsworth TR, Mulvaney RL (1995) Microscale determination of inorganic nitrogen in water and soil extracts. Commun Soil Sci Plant Analys 26:303–316
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn. Academic, London
Spiers GA, Gagnon D, Nason GE, Packee EC, Lousier JD (1986) Effects and importance of indigenous earthworms on decomposition and nutrient cycling in coastal forest ecosystems. Can J For Res 16:983–989
Springett JA, Syers JK (1979) The effect of earthworm casts on ryegrass seedlings. In: Crosby TK, Pottinger RP (eds) Proceedings of the 2nd Australasian conference on invertebrate ecology. Government Printer, Wellington, pp 47–49
Tibbett M (2000) Roots, foraging and the exploitation of soil nutrient patches: the role of mycorrhizal symbiosis. Funct Ecol 14:397–399
Tomati U, Grappelli A, Galli E (1988) The hormone-like effect of earthworm casts on plant growth. Biol Fertil Soils 5:288–294
van der Heijden MGA, Klironomos JN, Ursic M, Moutoglis P, Streitwolf-Engel R, Boller T, Wiemken A, Sanders IR (1998) Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 396:69–72
Vierheilig H, Coughlan AP, Wyss U, Piche Y (1998) Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Appl Environ Microbiol 64:5004–5007
Whalen JK, Sampedro L, Waheed T (2004) Quantifying surface and subsurface cast production by earthworms under controlled laboratory conditions. Biol Fertil Soils 39:287–291
White T, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M, Gelfand D, Sninsky J, White T (eds) PCR protocols: a guide to methods and applications. Academic, San Diego, pp 315–322
Wurst S, Dugassa-Gobena D, Langel R, Bonkowski M, Scheu S (2004) Combined effects of earthworms and vesicular–arbuscular mycorrhizas on plant and aphid performance. New Phytol 163:169–176
Yu X, Cheng J, Wong MH (2005) Earthworm-mycorrhiza interaction on Cd uptake and growth of ryegrass. Soil Biol Biochem 37:195–201
Zaller JG (2007) Effect of patchy distribution of soil nutrients on root morphology and biomass allocation of selected grassland species: experimental approach. Pol J Ecol 55:731–747
Zaller JG, Arnone JA (1997) Activity of surface-casting earthworms in a calcareous grassland under elevated atmospheric CO2. Oecologia 111:249–254
Zaller JG, Arnone JA (1999) Interactions between plant species and earthworm casts in a calcareous grassland under elevated CO2. Ecology 80:873–881
Zaller JG, Frank T, Drapela T (2011a) Soil sand content can alter effects of different taxa of mycorrhizal fungi on plant biomass production of grassland species. Europ J Soil Biol 47:175–181
Zaller JG, Heigl F, Grabmaier A, Lichtenegger C, Piller K, Allabashi R, Frank T, Drapela T (2011b) Earthworm-mycorrhiza interactions can affect the diversity, structure and functioning of establishing model grassland communities. PLoS ONE 6:e29293
Zaller JG, Saccani F, Frank T (2011c) Effects of earthworms and mycorrhizal fungi on the growth of the medicinal herb Calendula officinalis (Asteraceae). Plant Soil Environ 57:499–504
Zaller JG, Saxler N (2007) Selective vertical seed transport by earthworms: implications for the diversity of grassland ecosystems. Europ J Soil Biol 43:S86–S91
Zarea MJ, Ghalavand A, Goltapeh EM, Rejali F, Zamaniyan M (2009) Effects of mixed cropping, earthworms (Pheretima sp.), and arbuscular mycorrhizal fungi (Glomus mosseae) on plant yield, mycorrhizal colonization rate, soil microbial biomass, and nitrogenase activity of free-living rhizosphere bacteria. Pedobiol 52:223–235
Zhang H, Schrader S (1993) Earthworm effects on selected physical and chemical properties of soil aggregates. Biol Fertil Soils 15:229–234
Acknowledgments
We are grateful to Marcel van der Heijden, Dragana Bandian, and Sylvia Klaubauf for the constructive discussions during the course of the experiment and advice on the AMF detection using PCR. Lina Weissengruber, Lisa Kargl, Norbert Schuller, Janet Wissuwa, and Robert Corcoran helped during the harvest and postharvest sampling processing. The comments by three anonymous reviewers helped to improve this paper. This research was supported by the Austrian Science Fund (grant no. B20171-B16).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Zaller, J.G., Wechselberger, K.F., Gorfer, M. et al. Subsurface earthworm casts can be important soil microsites specifically influencing the growth of grassland plants. Biol Fertil Soils 49, 1097–1107 (2013). https://doi.org/10.1007/s00374-013-0808-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-013-0808-4