Abstract
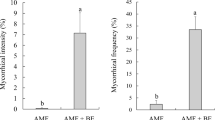
The present greenhouse study was undertaken to evaluate the effects of co-inoculating the ectomycorrhizal (ECM) fungus Boletus edulis with the mycorrhiza helper bacterium Bacillus cereus HB12 or HB59 on the growth and nutrient uptake of Pinus thunbergii. The inoculation with mycorrhiza helper bacterium significantly (P ≤ 0.05) increased the ectomycorrhizal colonization. Treatments with dual inoculum (the mycorrhiza helper bacterium plus mycorrhiza) significantly (P ≤ 0.05) increased the P. thunbergii growth. Bacteria–mycorrhizae interactions resulted in a great utilization of phosphate and potassium. The single inoculation resulted in a higher root activity than the control while the co-inoculation led to the highest root activity. The 6-CFDA staining assay showed that B. cereus enhanced fungal activity in ectomycorrhizal symbiosis. The results conclusively suggest that B. cereus isolated from the rhizosphere of P. thunbergii can potentially be used as individual inoculant or co-inoculated with ECM fungi to increase the production in sustainable ecological systems. These results support the potential use of B. cereus (HB12 or HB59) and B. edulis as mixed inoculants stimulating growth of P. thunbergii.








Similar content being viewed by others
References
Bending D, Poole EJ, Whipps JM, Read DJ (2002) Characterisation of bacteria from Pinus sylvestris-Suillus luteus mycorrhizas and their effects on root-fungus interactions and plant growth. FEMS Microbiol Ecol 39:219–227
Bowen GD, Theodorou C (1979) Interactions between bacteria and ectomycorrhizal fungi. Soil Biol Biochem 11:119–126
Brulé C, Frey-Klett P, Pierrat JC, Courrier S, Gérard F, Lemoine MC, Rousselet JL, Sommer G, Garbaye J (2001) Survival in the soil of the ectomycorrhizal fungus Laccaria bicolor and the effects of a mycorrhiza helper Pseudomonas fluorescens. Soil Biol Biochem 33:1683–1694
De Oliveira VL, Garbaye J (1989) Les microorganismes auxiliaires de l'etablissement des symbioses ectomycorrhiziennes. Eur J For Pathol 19:54–64
Deveau A, Palin B, Delaruelle C, Peter M, Kohler A, Pierrat JC, Sarniguet A, Garbaye J, Martin F, Frey-Klett P (2007) The mycorrhiza helper Pseudomonas fluorescens BBc6R8 has a specific priming effect on the growth, morphology and gene expression of the ectomycorrhizal fungus Laccaria bicolor S238N. New Phytol 175:743–755
Duponnois R, Garbaye J (1991) Effect of dual inoculation of Douglas fir with the ectomycorrhizal fungus Laccaria laccata and mycorrhization helper bacteria (MHB) in two bare-root forest nurseries. Plant Soil 138:169–176
Duponnois R, Kisa M (2006) The possible role of trehalose in the mycorrhiza helper effect. Can J Bot 84:1005–1008
Founoune H, Duponnois R, Bâ AM, Sall S, Branget I, Lorquin J, Neyra M, Chotte JL (2002) Mycorrhiza helper bacteria stimulate ectomycorrhizal symbiosis of Accacia holoserica with the Pisolithus albus. New Phytol 153:81–89
Frey-Klett P, Pierrat JC, Garbaye J (1997) Location and survival of mycorrhiza helper Pseudomonas fluorescens during establishment of ectomycorrhizal symbiosis between Laccaria bicolor and Douglas fir. Appl Environ Microbio 163:139–144
Garbaye J (1991) Biological interactions in the mycorhizospere. Experientia 47:370–375
Garbaye J (1994) Helper bacteria: a new dimension to themycorrhizal symbiosis. New Phytol 128:197–210
Garbaye J, Bowen GD (1989) Stimulation of ectomycorrhizal infection of Pinus radiata by some microorganisms associated with the mantle of ectomycorrhizas. New Phytol 112:383–388
Han GX, Zhang ZM, Wang GM, Mao PL, Su SJ, Xu QZ (2009) Growth dynamics and quantitative population characteristics of young trees in coastal Pinus thunbergii windbreak forest in northern Shandong Peninsula. Chin J Ecol 28:1013–1020
Hyde GJ, Ashford AE (1997) Vacuole motility and tubule-forming activity in Pisolithus tinctorius hyphae are modified by environmental conditions. Protoplasma 198:85–92
Insam H, Seewald MSA (2010) Volatile organic compounds. Biol Fertil Soils 46:199–214
Jackson ML (1973) Soil chemical analysis. Prentice-Hall of India Pvt. Ltd., New Delhi, India
Kataoka R, Futai K (2008) A new mycorrhizal helper bacterium, Ralstonia species, in the ectomycorrhizal symbiosis between Pinus thunbergii and Suillus granulatus. Biol Fertil 45:315–320
Maier A, Riedlinger J, Fiedler HP, Hampp R (2004) Actinomycetales bacteria from a spruce stand: characterization and effects on growth of root symbiotic and plant parasitic soil fungi in dual culture. Mycol Prog 18:185–190
Mcpartland JM, Robert CC, Watson DP (2000) Hemp diseases and pests: management and biological control: an advanced treatise. CABI Publishing, Wallingford
Minorsky PV (2004) On the inside. Plant Physiol 134(3):881–882
Poole EJ, Bending GD, Whipps JM, Read DJ (2001) Bacteria associated with Pinus sylvestris-Lactarius rufus ectomycorrhizas and their effects on mycorrhiza formation in vitro. New Phytol 151:743–751
Read DJ, Leake JR, Perez-Moreno J (2005) Erratum: mycorrhizal fungi as drivers of ecosystem processes in heathland and boreal forest biomes. Can J Bot 83:905–912
Ryan J, Garabet S, Harmsen K, Rashid A (1996) A soil and plant analysis manual adapted for the West Asia and North Africa Region. ICARDA, Allepo, Syria
Schrey S, Schellhammer M, Ecke M, Hampp R, Tarkka M (2005) Mycorrhiza helper bacterium Streptomyces AcH 505 induces differential gene expression in the ectomycorrhizal fungus Amanita muscaria. New Phytol 168:205–216
Shah S, Li JP, Barbara A, Moffatt GBR (1998) Isolation and characterization of ACC deaminase genes from two different plant growth promoting rhizobacteria. Can J Microbiol 44(9):833–843
Sheng JM, Wu XQ, Hou LL, Ying CX (2010) Isolation and identification of a MHB strain from the rhizosphere soil of Pinus thunbergi inoculated with Boletus edulis. Chin J Appl Environ Biol 16:701–704
Smith SE, Read DJ (1997) Mycorrhizal symbiosis, 2nd edn. Academic, New York
Stūrīte I, Henriksen TM, Breland TA (2005) Distinguishing between metabolically active and inactive roots by combined staining with 2, 3, 5-triphenyltetrazolium chloride and image colour analysis. Plant Soil 271:75–82
Sun MQ, Wu XQ, Ye JR (2007) Effects of ectomycorrhizal fungi on germination and growth of pines. Journal of Nanjing Forestry University (Natural Sciences Edition) 31:39–43
Tarkka MT, Piechulla B (2007) Aromatic weapons: truffles attack plants by the production of volatiles. New Phytol 175:381–383
Wang CX, Knill E, Bernard RG, Défago G (2000) Effect of transferring 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase genes into Pseudomonas fluorescens strain CHA0 and its gacA derivative CHA96 on their growth-promoting and disease-suppressive capacities. Can J Microbiol 46:898–907
Watanabe FS, Olsen S (1965) Test of an ascorbic acid method for determining phosphorus in water and NaHCO3 extract for soil. Soil Sci 21:677–678
Whipps JM (2004) Prospects and limitations formycorrhizas in biocontrol of root pathogens. Can J Bot 82:1198–1227
Zheng L, Wu XQ (2008) Morphology and activity of ectomycorrhizal fungi in vitro and in symbiont with pinus thunbergii. Journal of Plant Ecology (Chinese Version) 32:932–937
Zhu JJ, Li FQ, Takeshi M, Yutaka G (2002) Influence of thinning on regeneration in a costal Pinus thunbergii forest. Chin J Appl Ecol 13:1361–1367
Acknowledgments
This work is financially supported by Chinese Special Fund Project for the Scientific Research of the Forest Public Welfare Industry (201004061) and A Project founded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, XQ., Hou, LL., Sheng, JM. et al. Effects of ectomycorrhizal fungus Boletus edulis and mycorrhiza helper Bacillus cereus on the growth and nutrient uptake by Pinus thunbergii . Biol Fertil Soils 48, 385–391 (2012). https://doi.org/10.1007/s00374-011-0638-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-011-0638-1