Abstract
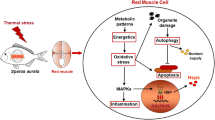
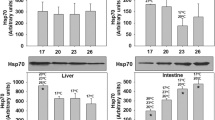
The winter syndrome in the gilthead sea bream Sparus aurata indicates that the species is exposed to critically low temperatures in Mediterranean aquaculture in winter. The present study of metabolic patterns and molecular stress responses during cold exposure was carried out to investigate this “disease”, in light of the recent concept of oxygen and capacity limited thermal tolerance. The metabolic profile of fuel oxidation was examined by determining the activities of the enzymes hexokinase (HK), aldolase (Ald), pyruvate kinase (PK), l-lactate dehydrogenase (l-LDH), citrate synthase (CS), malate dehydrogenase (MDH) and 3-hydroxyacyl CoA dehydrogenase (HOAD) in heart, red and white muscle after exposure to temperatures of 10, 14 and 18°C. Especially, the increase in LDH activity combined with the accumulation of l-lactate in tissues indicates that temperatures below 14°C are critical for Sparus aurata and stimulate the anaerobic component of metabolism. Increase in the activity of HOAD suggests that oxidation of free fatty acids might contribute to ATP turnover at low temperatures. The expression of Hsp70 and Hsp90 in all tissues examined revealed a cellular stress response during cooling below 18°C. In the light of winter temperatures in S. aurata cultures around 10°C, our data suggest that the fish are exposed to stressful conditions at the low end of their thermal tolerance window. These conditions likely impair the aerobic capacity of the fish, compromise the rates of growth and reproduction and may contribute to elicit pathological conditions.






Similar content being viewed by others
References
Abele D, Tesch C, Wencke P, Pörtner HO (2001) How do oxidative stress parameters relate to thermal tolerance in the Antarctic bivalve Yoldia eightsi? Antarct Sci 13:111–118
Ackerman PA, Forsyth RB, Mazur CF, Iwama GK (2000) Stress hormones and the cellular stress response in salmonids. Fish Physiol Biochem 23:327–336
Ali KS, Dorgai L, Abraham M, Hermesz E (2003) Tissue- and stressor-specific differential expression of two hsc70 genes in carp. Biochem Biophys Res Commun 307:503–509
Alves NR, Cordeiro O, Silva TS, Richard N, de Vareilles M, Marino G, Di Marco P, Rodrigues MP, Conceição ECL (2010) Metabolic molecular indicators of chronic stress in gilthead seabream (Sparus aurata) using comparative proteomics. Aquaculture 299:57–66
Bailey JR, Driedzic RW (1993) Influence of low temperature acclimation on fate of metabolic fuels in rainbow trout (Onchorhynchus mykiss) hearts. Can J Zool 71:2167–2173
Barton BA (2002) Stress in Fishes: A diversity of responses with particular reference to changes in circulating corticosteroids. Integ Comp Biol 42:517–525
Basu N, Nakano T, Grau EG, Iwama GK (2001) The effects of cortisol on heat shock protein 70 levels in two fish species. Gen Comp Endocrinol 124:97–105
Basu N, Todgham AE, Ackerman PA, Bibeau MR, Nakano K, Schulte PM, Iwama GK (2002) Heat shock Proteins genes and their functional significance in fish. Gene 295:173–183
Basu N, Kennedy CJ, Iwama GK (2003) The effects of stress on the association between hsp70 and the glucocorticoid receptor in rainbow trout. Comp Biochem Physiol 134:655–663
Bovo G, Borghesan F, Comuzzi M, Ceschias G, Giorgetti G (1995) “Winter disease” in orata di allevamento: observazioni preliminary. Boll Soc Ital Patol Ittica 17:2–11
Dean JM (1969) The metabolism of tissues of thermally acclimated trout (Salmo gairdneri). Comp Biochem Physiol 29:185–196
Deane EE, Woo NYS (2005) Cloning and characterization of the hsp70 multigene family from silver sea bream: Modulated gene expression between warm and cold temperature acclimation. BBRC 330:776–783
Desaulniers N, Moerland TS, Sidell BD (1996) High lipid content enhances the rate of oxygen diffusion through fish skeletal muscle. Am J Physiol 271:R42–R47
Doimi M (1996) A new winter disease in sea bream (Sparus aurata): a preliminary report. Bull Eur Assoc Fish Pathol 16:17–18
Driedzic RW, Almeida-Val VMF (1996) Enzymes of cardiac energy metabolism in Amazonian teleosts and fresh-water stingray (Potamotrygon hystrix). J Exp Zool 274:327–333
Driedzic RW, Sidell DB, Stowe D, Branscombe R (1987) Matching of vertebrate cardiac energy demand to energy metabolism. Am J Physiol 252:R930–R937
Egginton S, Sidell BD (1989) Thermal acclimation induces adaptative changes in subcellular structure of fish skeletal muscle. Am J Physiol 256:R1–R9
Egginton S, Cordiner S, Skilbeck C (2000) Thermal compensation of peripheral oxygen transport in skeletal muscle of seasonally acclimatised trout. Am J Physiol 279:R375–R388
Feder ME, Hofmann GE (1999) Heat-shock proteins, molecular chaperones and the stress response: evolutionary and ecological physiology. Annu Rev Physiol 61:243–282
Giardina B, Mordente A, Zappacosta B, Calla C, Colacicco L, Gozzo ML, Lippa S (1998) The oxidative metabolism of Antarctic fish: some peculiar aspects of cold adaptation. In: Prisco G, Pisano E, Clarke A (eds) Fishes of Antarctica. A biological overview. Springer, Italy, pp 129–138
Guderley H (1990) Functional significance of metabolic responses to thermal acclimation in fish muscle. Am J Physiol Regul Integr Comp Physiol 28:R245–R252
Guderley H (2004) Metabolic responses to low temperature in fish muscle. Biol Rev 79:409–427
Guderley H, Gawlicka A (1992) Qualitative modification of muscle metabolic organization with thermal acclimation of rainbow trout, Corhynchus mykiss. Fish Physiol Biochem 10:123–132
Hochachka PW, Somero GN (2002) Biochemical adaptation. Princeton University Press, Princeton
Hofmann GE, Buckley BA, Place SP, Zippay ML (2002) Molecular chaperones in ectothermic marine animals: biochemical function and gene expression. Integr Comp Biol 42:808–814
Ibarz A, Fernádez-Borràs J, Blasco J, Gallardo AM, Sanchez J (2003) Oxygen consumption and feeding rates of gilthead sea bream (Sparus aurata) reveal lack of acclimation to cold. Fish Physiol Biochem 29:313–321
Ibarz A, Blasco J, Beltrán M, Gallardo MA, Sánchez J, Sala R, Fernández-Borràs J (2005) Cold-induced alterations on proximate composition and fatty acid profiles of several tissues in gilthead sea bream (Sparus aurata). Aquaculture 249:477–486
Ibarz A, Blasco J, Sala-Rabanal M, Gallardo AM, Redondo A, Fernádez-Borràs J (2007) Metabolic rate and tissue reserves in gilthead sea bream (Sparus aurata) under thermal fluctuations and fasting and their capacity for recovery. Can J Fish Aquat Sci 64:1034–1042
Ibarz A, Padrόs F, Gallardo AM, Fernádez-Borràs J, Blasco J, Tort L (2010) Low-temperature challenges to gilthead sea bream culture: review of cold-induced alterations and ‘Winter Syndrome’. Rev Fish Biol Fisheries. doi:10.1007/s11160-010-9159-5
Iwama GK, McGeer JC, Pawluk MP (1989) The effects of five fish anesthetics on acid–base balance, hematocrit, blood gases, cortisol, and adrenaline in rainbow trout. Can J Fish Aquat Sci 67:2065–2073
Iwama GK, Thomas P, Vijayan MM, Forsyth RB (1998) Stress proteins expression in fish. Rev Fish Biol Fish 8:35–56
Iwama GK, Vijayan MM, Forsyth RB, Ackerman PA (1999) Heat shock proteins and physiological stress in fish. Am Zool 39:901–909
Iwama GK, Afonso LOB, Todgham A, Ackerman P, Nakano K (2004) Are hsps suitable for indicating stressed states in fish? J Exp Biol 20:15–19
Johnston IA, Moon TW (1980a) Endurance exercise training in the fast and slow muscles of a teleost fish (Pollachius virens). J Comp Physiol 135:147–156
Johnston IA, Moon TW (1980b) Exercise training in skeletal muscle of brook trout (Salvelinus fontinalis). J Exp Biol 87:177–194
Jones PL, Sidell BD (1982) Metabolic responses of striped bass (Morone saxatilis) to temperature acclimation. II. Alterations in metabolic carbon sources and distributions of fibre types in locomotory muscle. J Exp Zool 219:163–171
Ju Z, Dunham RA, Liu Z (2002) Differential gene expression in the brain of channel catfish (Ictalurus punctatus) in response to cold acclimation. Mol Genet Genomics 268:87–95
Kassahn K, Crozier RH, Pörtner HO, Caley MJ (2009) Animal performance and stress: responses and tolerance limits at different levels of biological organisation. Biol Rev 84:277–292
Kleckner NW, Sidell BD (1985) Comparison of maximal activities of enzymes from tissues of thermally acclimated and naturally acclimatized chain pickerel (Esox niger). Physiol Zool 58:18–28
Lowry OH, Passonneau JV (1972) A flexible system of enzymatic analysis. Academic Press, New York
Montero D, Izquierdo MS, Tort L, Robaina L, Vergara JM (1999) High stocking density produces crowding stress altering some physiological and biochemical parameters in gilthead seabream, Sparus aurata, juveniles. Fish Physiol Biochem 20:53–60
Moon TW, Mommsen TP (1987) Enzymes of intermediary metabolism in tissues of little skate (Raja erinacea). J Exp Zool 244:9–15
Petersen NS, Young P, Burton V (1990) Heat-shock messenger-RNA accumulation during recovery from cold shock in Drosophila melanogaster. Inst Biochem 20:679–684
Place SP, Hofmann GE (2005) Function and expression of the molecular chaperone HSP70 in Antarctic fishes. Faseb J 19:A214–A214
Place SP, Zippay ML, Hofmann GE (2004) Constitutive roles for inducible genes: evidence for the alteration in expression of the inducible hsp70 gene in Antarctic notothenioid fishes. Am J Physiol Regulat Integr Comp Physiol 287:R429–R436
Pörtner HO (2001) Climate change and temperature dependent biogeography: oxygen limitation of thermal tolerance in animals. Naturwissenschaften 88:137–146
Pörtner HO (2002) Climate change and temperature dependent biogeography: systemic to molecular hierarchies of thermal tolerance in animals. Comp Biochem Physiol 132A:739–761
Pörtner HO, Farrell AP (2008) Physiology and climate change. Science 322:690–692
Pörtner HO, Knust R (2007) Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science 315:95–97
Pörtner HO, Langenbuch M, Reipschläger A (2004) Biological impact of elevated ocean CO2 concentrations: lessons from animal physiology and earth history? J Oceanogr 60:705–718
Pörtner HO, Langenbuch M, Michaelidis B (2005) Synergistic effects of temperature extremes, hypoxia and increases in CO2 on marine animals: from earth history to global change J Geophys Res Oceans:110, C09S10. doi:10.1029/2004JC002561
Pörtner HO, Farrell AP, Knust R, Lannig G, Mark FC, Storch D (2009) Adapting to climate change—response. Science 323:876–877
Requena A, Fernandez J, Planas J (1997) The effects of a temperature rise on oxygen consumption and energy budget in gilthead sea bream. Aquac Int 5:415–426
Rotllant J, Balm PHM, Wendelaar-Bonga SE et al (2000) A drop in ambient temperature results in a transient reduction of interrenal ACTH responsiveness in the gilthead sea bream (Sparus aurata, L.). Comp Biochem Physiol 23:265–273
Sarusic G (1999) Clinical signs of the winter disease phenomenon in the sea bream (Sparus aurata, L.). Bull Eur Ass Fish Pathol 19:113
Sephton DH, Driedzic RW (1991) Effect of acute and chronic temperature transition on enzymes of cardiac metabolism in white perch (Morone americana), yellow perch (Perm flauescens), and smallmouth bass (Micropterus dolumieui). Can J Zool 69:258–262
Sephton DH, Bailey J, Driedzic RW (1990) Impact of acute temperature transition on enzyme activity levels, oxygen consumption, and exogenous fuel utilization in sea raven (Hemitripterus americanus) hearts. J Comp Physiol 160B:511–518
Sidell BD, Driedzic W, Stowe DB, Johnston IA (1987) Biochemical correlations of power development and metabolic fuel preferenda in fish hearts. Physiol Zool 60:221–232
Singer TD, Ballantyne JS (1989) Absence of extrahepatic lipid oxidation in a freshwater elasmobranch, the dwarf stingray (Potamotrygon megdalenae): evidence from enzyme activities. J Exp Zool 251:355–360
Somero GN (1995) Proteins and temperature. Ann Rev Physiol 57:43–68
St-Pierre J, Pierre-Mathieu C, Guderley H (1998) Relative contribution of quantitative and qualitative changes in mitochondria to metabolic compensation during seasonal acclimatisation of rainbow trout Oncorhynchus mykiss. J Exp Biol 201:2961–2970
Todgham AEH, Hoaglund EA, Hofmann GE (2007) Is cold the new hot? Elevated ubiquitin-conjugated protein levels in tissues of Antarctic fish as evidence for cold-denaturation in vivo. J Comp Physiol B 177:857–866. doi:10.1007/s00360-007-0183-2
Tort L, Padrós F, Rotllant J, Crespo S (1998) Winter syndrome in the gilthead sea bream Sparus aurata. Immunological and histopathological features. Fish Shellfish Immunol 8:37–47
Tsikliras CA, Torre M, Stergiou IK (2005) Feeding habits and trophic level of round sardimella (Sardinella aurita) in the northeastern Mediterranean (Aegean Sea, Greece). J Biol Res 3:67–75
Van Dijk PLM, Tesch C, Hardewig I, Pörtner HO (1999) Physiological disturbances at critically high temperatures: a comparison between stenothermal Antarctic and eurythermal temperate eelpouts (Zoarcidae). J Exp Biol 202:3611–3621
Viarengo A, Canesi L, Gracia Martinez P, Peters LD, Livingstone DR (1995) Pro-oxidant processes and antioxidant defence systems in the tissues of the Antarctic scallop (Adamussium colbecki) compared with the Mediterranean scallop (Pecten jacobaeus). Comp Biochem Physiol B 111:119–126
Viarengo A, Abele-Oeschger D, Burlando B (1998) Effects of low temperature on prooxidant processes and antioxidant defence systems in marine organisms. In: Pörtner H-O, Playle RC (eds) Cold ocean physiology. Cambridge University Press, Cambridge, pp 213–235
Weber JM, Haman F (1996) Pathways for metabolic fuels and oxygen-in high performance fish. Comp Biochem Physiol 113 A:33–38
Yocum GD, Joplin KH, Denlinger DL (1991) Expression of heat-shock proteins in response to high and low-temperature extremes in diapausing pharate larvae of the gypsy-moth, Lymantria dispar. Arch Inst Biochem Physiol 18:239–249
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Heldmaier.
Rights and permissions
About this article
Cite this article
Kyprianou, TD., Pörtner, H.O., Anestis, A. et al. Metabolic and molecular stress responses of gilthead seam bream Sparus aurata during exposure to low ambient temperature: an analysis of mechanisms underlying the winter syndrome. J Comp Physiol B 180, 1005–1018 (2010). https://doi.org/10.1007/s00360-010-0481-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-010-0481-y