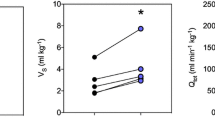
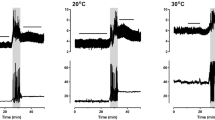
Abstract
Venous blood flow was measured for the first time in a cephalopod. Blood velocity was determined in the anterior vena cava (AVC) of cuttlefish S. officinalis with a Doppler, while simultaneously, ventilatory pressure oscillations were recorded in the mantle cavity. In addition, magnetic resonance imaging (MRI) was employed to investigate pulsatile flow in other major vessels. Blood pulses in the AVC are obligatorily coupled to ventilatory pressure pulses, both in frequency and phase. AVC peak blood velocity (vAVC) in animals of 232 (± 30 SD) g wet mass at 15°C was found to be 14.2 (± 7.1) cm s−1, AVC stroke volume (SVAVC) was 0.2 (± 0.1) ml stroke−1, AVC minute volume (MVAVC) amounted to 5.5 (± 2.8) ml min−1. Intense exercise bouts of 1–2 min resulted in 2.2-fold increases in MVAVC, enabled by 1.6-fold increments in both, AVC pulse frequency (f AVC) and vAVC. As increases in blood flow occurred delayed in time by 1.7 min with regard to exercise periods, we concluded that it is not direct mantle cavity pressure conveyance that drives venous return in this cephalopod blood vessel. However, during jetting at high pressure amplitude (> 1 kPa), AVC blood flow and mantle cavity pressure pulse shapes completely overlap, suggesting that under these conditions, blood transport must be driven passively by mantle cavity pressure. MRI measurements at 15°C also revealed that under resting conditions, f AVC and ventilation frequency (f V) match at 31.6 (± 2.1) strokes min−1. In addition, rates of pulsations in the cephalic artery and in afferent branchial vessels did not significantly differ from f AVC and f V. It is suggested that these adaptations are beneficial for high rates of oxygen extraction observed in S. officinalis and the energy conserving mode of life of the cuttlefish ecotype in general.






Similar content being viewed by others
References
Aitken JP, O’Dor RK (2004) Respirometry and swimming dynamics of the Australian cuttlefish Sepia apama. Mar Freshw Behav Physiol 37:217–234
Aitken JP, O’Dor RK, Jackson GD (2005) The secret life of the giant cuttlefish Sepia apama (Cephalopoda). Behaviour and energetics in nature revealed through radio acoustic positioning and telemetry (RAPT). J Exp Mar Biol Ecol 320:77–91
Bert P (1867) Mémoire sur la physiologie de la Seiche. Mém Soc Sci phys Nat Bordeaux 5:115
Bock C, Sartoris FJ, Pörtner HO (2002) In vivo MR spectroscopy and MR imaging on non anaesthetized marine fish: techniques and first results. Magn Reson Imaging 20:165–172
Bone Q, Brown ER, Travers G (1994) On the respiratory flow in the cuttlefish Sepia officinalis. J Exp Biol 194:153–165
Bourne GB (1982) Blood pressure in the squid, Loligo pealei. Comp Biochem Physiol 72A:23–27
Bourne GB, Redmond JT, Johansen K (1978) Some aspects of heemodynamics in Nautilus pompilius. J Exp Zool 205:63–70
Denton EJ, Gilpin-Brown JB (1961) The effect of light on the buoyancy of the cuttlefish. J Mar Biolog Assoc UK 41:343–350
De Wilde J (1956) Koordination im Gefässsystem von Octopus vulgaris. L Pubbl Staz Zool Napoli 28:359–366
Fiedler A (1992) Die Rolle des venösen Füllungsdrucks bei der Autoregulation der Kiemenherzen von Sepia officinalis L. (Cephalopoda). Zool jb Physiol 96:265–278
Haase A (1990) Snapshot FLASH MRI. Applications to T1, T2 and chemical shift imaging. Magn Reson Med 13:77–89
Joaquim N, Wagner GN, Gamperl AK (2004) Cardiac function and critical swimming speed of the winter flounder (Pleuronectes americanus) at two temperatures. Comp Biochem Physiol A 138:277–285
Johansen K (1965) Cardiac output in the large cephalopod Octopus dofleini. J Exp Biol 42:475–480
Johansen K, Martin AW (1962) Circulation in the cephalopod, Octopus dofleini. Comp Biochem Physiol 5:161–176
Johansen K, Brix O, Lykkeboe G (1982) Blood gas transport in the cephalopod, Sepia officinalis. J Exp Biol 99:331–338
King AJ, Henderson SM, Schmidt MH, Cole AG, Adamo SA (2005) Using ultrasound to understand vascular and mantle contributions to venous return in the cephalopod Sepia officinalis Linneaus. J Exp Biol 208:2071–2082
Madan JJ, Wells MJ (1996) Cutaneous respiration in Octopus vulgaris. J Exp Biol 199:2477–2483
Mather JA, O’Dor RK (1991) Foraging strategies and predation risk shape the natural history of juvenile Octopus vulgaris. Bull Mar Sci 49:256–269
Mark FC, Bock C, Pörtner HO (2002) Oxygen-limited thermal tolerance in Antarctic fish investigated by MRI and 31P-MRS. Am J Physiol 283:R1254–R1262
Messenger JB, Nixon M, Ryan KP (1985) Magnesium chloride as an anaesthetic for cephalopods. Comp Biochem Physiol C 82(1):203–205
Melzner F, Bock C, Pörtner HO (2006a) Critical temperatures in the cephalopod Sepia officinalis investigated using in vivo 31P NMR spectroscopy. J Exp Biol 209:891–906
Melzner F, Bock C, Pörtner HO (2006b) Temperature dependent oxygen extraction from the ventilatory current and the costs of ventilation in the cephalopod Sepia officinalis. J Comp Physiol B DOI 10.1007/s00360–006–0084–9
Mislin H (1950) Nachweis einer reflektorischen Regulation des peripheren Kreislaufs der Cephalopoden. Experientia 6:467–468
Mislin H (1966) Ueber die Beziehungen zwischen Atmung und Kreislauf bei Cephalopoden (Sepia officinalis L.). Synchronregistrierung von Elektrokardiogramm (Ekg) und Atembewegungen am schwimmenden Tier. Verh Dtsch Zool Ges 175–181
Mislin H, Kauffmann M (1948) Der aktive Gefässpuls in der Armschrimhaut der Cephalopoden. Rev Suisse Zool 55:267–271
Pörtner HO (1994) Coordination of metabolism, acid-base regulation and haemocyanin function in cephalopods. In: Pörtner HO, O’Dor RK, Macmillan DL (eds) Physiology of cephalopod molluscs: lifestyle and performance adaptations. Gordon and Breach, Basel pp 131–148
Schipp R (1987a) The blood vessels of cephalopods. A comparative morphological and functional survey. Experientia 43:525–537
Schipp R (1987b) General morphological and functional characteristics of the cephalopod circulatory system. An introduction. Experientia 43:474–477
Shadwick RE (1994) Mechanical oraganization of the mantle and circulatory system of cephalopods. In: Pörtner HO, O’Dor RK, Macmillan DL (eds) Physiology of cephalopod molluscs: lifestyle and performance adaptations. Gordon and Breach, Basel, pp 69–85
Shadwick RE, Nilsson EK (1990) The importance of vascular elasticity in the circulatory system of the cephalopod Octopus vulgaris. J Exp Biol 152:471–484
Shadwick RE, O’Dor RK, Gosline JM (1990) Respiratory and cardiac function during exercise in squid. Can J Zool 68:792–798
Smith LS (1962) The role of venous peristalsis in the arm circulation of Octopus dofleini. Comp Biochem Physiol 7:269–275
Storey KB, Storey JM (1979) Octopine metabolism in the cuttlefish, Sepia officinalis: octopine production by muscle and ist role as an aerobic substrate for non-muscular tissues. J Comp Physiol 131:311–319
Tompsett DH (1939) Sepia. LMBC memoirs, Liverpool University Press, 184 pp
Skramlik Ev (1941) Über den Kreislauf bei den Weichtieren. Ergebn Biol 18:88–286
Webber DM, Boutilier RG, Kerr SR (1998) Cardiac output as a predictor of metabolic rate in cod Gadus morhua. J Exp Biol 204:3561–3570
Wells MJ (1994) The evolution of a racing snail. In: Pörtner HO, O’Dor RK, Macmillan DL (eds) Physiology of cephalopod molluscs: lifestyle and performance adaptations. Gordon and Breach, Basel pp 1–11
Wells MJ, Smith PJS (1987) The performance of the octopus circulatory system: a triumph of engineering over design. Experientia 43:487–499
Wells MJ, Wells J (1983) The circulatory response to acute hypoxia in Octopus. J Exp Biol 104:59–71
Wells MJ, Wells J (1986). Blood flow in acute hypoxia in a cephalopod. J Exp Biol 122:345–353
Wells MJ, Wells J (1991). Is Sepia really an octopus? In: Boucaud-Camou E (ed), La Seiche, 1st International symposium on the cuttlefish Sepia. Centre de publications, Universite de Caen, pp 77–92
Wells MJ, O’Dor RK, Mangold K, Wells J (1983) Oxygen consumption and movement by Octopus. Mar Behav Physiol 9:289–303
Wells MJ, Duthie GG, Houlihan DF, Smith PJS, Wells J (1987). Blood flow and pressure changes in exercising octopuses (Octopus vulgaris). J Exp Biol 131:175–187
Wells MJ, Hanlon RT, Lee PG, Dimarco FP (1988). Respiratory and cardiac performance in Lolliguncula brevis (Cephalopoda, Myopsida): the effects of activity, temperature and hypoxia. J Exp Biol 138:17–36
Acknowledgments
This study was carried out in support of the project: The cellular basis of standard and active metabolic rate in the free-ranging cephalopod, Sepia officinalis (NER/A/S/2002/00812). The authors wish to thank Timo Hirse and Rolf Wittig for excellent technical support, Raymond and Marie-Paule Chichery (Université de Caen) for providing cuttlefish eggs in 2002 and 2003 and all student helpers that were engaged in raising the animals in our lab. Special thanks to Alison King for helpful and stimulating discussions on the subject of venous return in cephalopod and to two anonymous reviewers whose excellent criticism improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Heldmaier
Rights and permissions
About this article
Cite this article
Melzner, F., Bock, C. & Pörtner, HO. Coordination between ventilatory pressure oscillations and venous return in the cephalopod Sepia officinalis under control conditions, spontaneous exercise and recovery. J Comp Physiol B 177, 1–17 (2007). https://doi.org/10.1007/s00360-006-0104-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-006-0104-9