Abstract
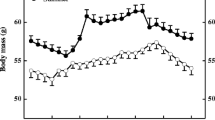
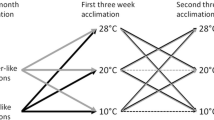
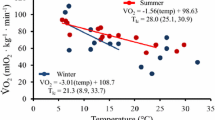
Seasonal adjustments in body mass and thermogenesis are important for the survival of small mammals during acclimatization in the temperate zone. To determine the contributions of short photoperiod and cold temperatures to seasonal changes in thermogenesis and body mass in Mongolian gerbils (Meriones unguiculatus), body mass, basal metabolic rate (BMR), nonshivering thermogenesis (NST), energy intake and energy digestibility were determined in seasonally acclimatized and laboratory acclimated animals. Body mass showed significant seasonal changes and decreased to a minimum in winter. Both BMR and NST increased in winter, and these changes were mimicked by exposing animals to short photoperiod or cold temperatures in the animal house. Digestible energy intake also increased significantly in winter, and also during exposure of housed animals to both short photoperiod and cold. These results suggest that Mongolian gerbils overcome winter thermoregulatory challenges by increasing energy intake and thermogenesis, and decreasing body mass to reduce total energy requirements. Short photoperiod and cold can serve as effective environmental cues during seasonal acclimatization.


Similar content being viewed by others
References
Bacigalupe LD and Bozinovic F (2002) Design, limitations and sustained metabolic rate: lessons from small mammals. J Exp Biol 205:2963–2970
Bartness TJ and Wade GN (1984) Photoperiodic control of body weight and energy metabolism in Syrian hamsters (Mesocricetus auratus): role of pineal gland, melatonin, gonads, and diet. Endocrinology 114:492–498
Bartness TJ and Wade GN (1985) Photoperiodic control of seasonal body weight cycles in hamsters. Neurosci Biobehav Rev 9:599–612
Bartness TJ, Demas GE, Song CK (2002) Seasonal changes in adiposity: the roles of the photoperiod, melatonin and other hormones, and sympathetic nervous system. Exp Biol Med 227:363–376
Bozinovic F, Novoa FF, Veloso C (1990) Seasonal changes in energy expenditure and digestive tract of Abrothrix andinus (Cricetidae) in the Andes Range. Physiol Zool 63:1216–1231
Bozinovic F, Bacigalupe LD, Vasquez RA, Visser GH, Veloso C, Kenagy GJ (2004) Cost of living in free-ranging degus (Octodon degus): seasonal dynamics of energy expenditure. Comp Biochem Physiol A 137:597–604
Dark J, Zucker I, Wade GN (1983) Photoperiodic regulation of body mass, food intake, and reproduction in meadow voles. Am J Physiol 245:R334–R338
Del Valle JC and Busch C (2003) Body composition and gut length of Akodon azarae (Muridae: Sigmodontinae): relationship with energetic requirements. Acta Theriol 48(3):347–357
Gorecki A (1975) Kalabukhov-Skvortsov respirometer and resting metabolic rate measurement. In: Grodzinski W, Klekowski R, Duncan ZA (eds) Methods for ecological energetics. Blackwell, Oxford, pp 309–313
Grodzinski W, Wunder BA (1975) Ecological energetics of small mammals. In: Golley FB, Petrusewicz K, Ryszkowski L (eds) Small mammls: their productivity and population dynamics. Cambridge University Press, Cambridge, pp 173–204
Hayssen V, Lacy RC (1985) Basal metabolic rate in mammals: taxonomic differences in the allometry of BMR and body mass. Comp Biochem Physiol 81A:741–754
Heldmaier D (1971) Zitterfreie warmebidung und körpergröbe säugetieren. Z Vergl Physiol 73:222–248
Heldmaier G and Steinlechner S (1981) Seasonal control of energy requirements for thermoregulation in the Djungarian hamster (Phodopus sungorus), living in natural photoperiod. J Comp Physiol B 142:429–437
Heldmaier G, Steinlechner S, Rafael J, and Vsiansky P (1981) Photoperiodic control and effects of melatonin on nonshivering thermogenesis and brown adipose tissue. Science 212:917–919
Heldmaier G, Steinlechner S, Rafael J, Latteier B (1982) Photoperiod and ambient temperature as environmental cues for seasonal thermogenic adaptation in the Djungarian hamster, Phodopus sungorus. Int J Biometeorol 26:339–345
Heldmaier G, Steinlechner S, Rafael J (1982) Nonshivering thermogenesis and cold resistance during seasonal acclimation in the Djungarian hamster. J Comp Physiol B 149:1–9
Iverson SL, Turner BN (1974) Winter weight dynamics in Microtus pennsylvanicus. Ecology 55:1030–1041
Jackson DM, Hambly C, Trayhurn P, Speakman JR (2001) Associations between energetics and over-winter survival in the short-tailed field vole Microtus agrestis. J Anim Ecol 70:633–640
Jansky L, Haddad G, Pospisilova D, Dvorak P (1986) Effect of external factors on gonadal activity and body mass of male golden hamsters (Mesocricetus auratus). J Comp Physiol B 156:717–725
Klaus S, Heldmaier G, Ricquier D (1988) Seasonal acclimation of bank voles and wood mice: nonshivering thermogenesis and thermogenic properties of brown adipose tissue mitochondria. J Comp Physiol B 158:157–164
Klingenspor M, Klaus S, Wiesinger H, Heldmaier G (1989) Short photoperiod and cold activate brown fat lipoprotein lipase in the Djungarian hamster. Am J Physiol 257:R1123–R1127
Klingenspor M, Niggemann H, Heldmaier G (2000) Modulation of leptin sensitivity by short photoperiod acclimation in the Djungarian hamster, Phodopus sungorus. J Comp Physiol B 170:37–43
Knopper LD and Boily P (2000) The energy budget of captive Siberian hamster, Phodopus sungorus, exposed to photoperiod changes: mass loss is caused by a voluntary decrease in food intake. Physiol Biochem Zool 73:517–522
Kronfeld-Schor N, Haim A, Dayan T, Zisapel N, Klingenspor M, Heldmaier G (2000) Seasonal thermogenic acclimation of diurnally and nocturnally active desert spiny mice. Physiol Biochem Zool 73:37–44
Li QF, Sun RY, Huang CX, Wang ZK, Liu XT, Hou JJ, Liu JS, Cai LQ, Li N, Zhang SZ, Wang Y (2001) Cold adaptive thermogenesis in small mammals from different geographical zones of China. Comp Biochem Physiol A 129:949–961
Li XS, Wang DH (2005) Regulation of body weight and thermogenesis in seasonally acclimatized Brandt’s voles (Microtus brandti). Horm Behav (in press)
Li XS, Wand DH, Yang JC (2003) Effect of photoperiod on body weight and energy metabolism in Brandt’s voles (Microtus brandti) and Mongolian gebils (Meriones unguiculatus) (in Chinese with English summary). Acta Theriol Sin 23:304–311
Li XS, Wang DH, Yang M (2004) Effects of cold acclimation on body weight, serum leptin level, energy metabolism and thermogenesis in the Mongolian gerbil (Meriones unguiculatus) (in Chinese with English summary). Acta Zool Sin 50:334–340
Liu H, Wang DH, Wang ZW (2002) Maximum metabolizable energy intake in the Mongolian gerbil (Meriones unguiculatus). J Arid Environ 52:405–411
Liu H, Wang DH, Wang ZW (2003) Energy requirement during reproduction in female Brandt’s voles (Microtus brandti). J Mammal 84(4):1410–1416
Liu JS, Wang DH, Sun RY (2004) Metabolism and thermoregulation in three species of rodent from Northeastern China. J Therm Biol 29(3):177–183
Merritt JF (1995) Seasonal thermogenesis and body changes in body mass of masked shrews, Sorex cinereus. J Mammal 76:1020–1035
Merritt JF and Zegers DA (1991) Seasonal thermogenesis and body mass dynamics of Clethrionomys gapperi. Can J Zool 69:2771–2777
Merritt JF, Zegers DA, Rose LR (2001) Seasonal thermogenesis of southern flying squirrels (Glaucomys volans). J Mammal 82:51–64
Nagy TR (1993) Effects of photoperiod history and temperature on male collared lemmings, Dicrostonyx groenlandicus. J Mammal 74:990–998
Nagy TR and Negus NC (1993) Energy acquisition and allocation in male collared lemmings (Dicrostonyx groenlandicus): effects of photoperiod, temperature, and diet quality. Physiol Zool 66:737–560
Nagy TR, Gower BA, Stetson MH (1995) Endocrine correlates of seasonal body weight dynamics in the collared lemming (Dicrostonyx groenlandicus). Am Zool 35:246–258
Nespolo RF, Opazo JC, Rosenmann M, Bozinovic F (1999) Thermal acclimation, maximum metabolic rate and nonshivering thermogenesis in Phyllotis xanthopygus (Rodentia) inhabiting the Andean range. J Mammal 80:742–748
Peacock WL, Krol E, Moar KM, McLaren JS, Mercer JG, Speakman JR (2004) Photoperiodic effects on body mass, energy balance and hypothalamic gene expression in the bank vole. J Exp Biol 207:165–177
Pei YX, Wang DH, Hume ID (2001) Effects of dietary fibre on digesta passage, nutrient digestibility, and gastrointestinal tract morphology in the granivorous Mongolian gerbils (Meriones unguiculatus). Physiol Biochem Zool 74:742–749
Powell CS, Blaylock ML, Wang R, Hunter HL, Johanning GL, Nagy TR (2002) Effects of energy expenditure and Ucp1 on photoperiod-induced weight gain in collard lemmings. Obes Res 10:541–550
Rosenmann M, Morrison P, Feist D (1975) Seasonal changes in the metabolic capacity of red-backed voles. Physiol Zool 48:303–310
Song ZG, Wang DH (2001) Maximum energy assimilation rate in Brandt’s vole (Microtus brandti) from Inner Mongolian grassland (in Chinese with English summary) . Acta Theriol Sin 21:271–278
Song, ZG, Wang DH (2002) Relationships between metabolic rates and body composition in the Mongolian gerbil (Meriones unguiculatus) (in Chinese with English summary). Acta Zoologica Sinica 48:445–451
Song ZG, Wang DH (2003a) Metabolism and thermoregulation in the striped hamster Cricetulus barabensis. J Therm Biol 28:509–514
Song, ZG, Wang DH (2003b) Relationships between metabolic rate and body composition in Brandt’s voles (Microtus brandti) (in Chinese with English summary). Acta Theriol Sin 23:230–234
Speakman (1996) Energetics and the evolution of body size in small terrestrial mammals. Symp Zool Soc Lond 69:63–81
Speakman JR, Johnson MS (2000) Relationships between resting metabolic rate and morphology in lactating mice: what tissues are the major contributors to resting metabolism?. In: Heldmaier G, Klingenspor M (eds) Life in the cold. Springer, Berlin Heidelberg New York, pp 497–486
Steinlechner S, Heldmaier G, Becker H (1983) The seasonal cycle of body weight in the Djungarian hamster: photoperiod control and the influence of starvation and melatonin. Oecologia 60:401–405
Veloso C and Bozinovic F (2000) Interplay between acclimation time and diet quality on basal metabolic rate in females of degus Octodon degus (Rodentia: octodontidae). J Zool 252:531–533
Voltura MB (1996) Seasonal variation in body composition and gut capacity of the prairie vole (Microtus ochrogaster). Can J Zool 75:1714–1719
Voltura MB and Wunder BA (1998) Effects of ambient temperature, diet quality, and food restriction on body composition dynamics of the prairie vole, Microtus ochrogaster. Physiol Zool 71:321–328
Wang DH, Wang ZW (1996) Seasonal variations on thermogenesis and energy requirements of plateau pikas Ochotona curzoniae and root voles Microtus oeconomus. Acta Theriol 41:225–236
Wang DH, Sun RY, Wang ZW, Liu JS (1999) Effects of temperature and photoperiod on thermogenesis in plateau pikas (Ochotona curzoniae) and root voles (Microtus oeconomus). J Comp Physiol B 169:77–83
Wang DH, Wang YS, Wang ZW (2000) Metabolism and thermoregulation in the Mongolian gerbils Meriones unguiculatus. Acta Theriol 45(2):183–192
Wang DH, Wang ZW, Wang YS, Yang JC (2003) Seasonal changes of thermogenesis in Mongolian gerbils (Meriones unguiculatus) and Brandt’s voles (Microtus brandti) (Abstract). Comp Biochem and Physiol A 134:S96
Wunder BA, Gettinger RD (1996) Effects of body mass and temperature acclimation on the nonshivering thermogenic response of small mammals. In: Geiser F, Hulbert AJ, Nicol SC (eds) Adaptions to the cold: tenth international hibernation symposium. University of New England Press, Armidale, pp 131–139
Zhang ZB, Wang ZW (1998) Ecology and management of rodent pests in agriculture (In Chinese with English summary). Ocean Press, Beijing
Zhao ZJ,Wang DH (2005) Short photoperiod enhances thermogenic capacity in Brandt's voles. Physiol Behav 85(2):143-149
Acknowledgements
We are grateful to Professor Ian Hume, University of Sydney, Australia, for his helpful comments and correcting language errors. This study was supported by grants from the National Natural Science Foundation of China (No. 30170151 and 30430140) and the Chinese Academy of Sciences (No. KSCX2-SW-103) to DHW, and the State Key Laboratory of Integrated Management of Pest Insects and Rodents (IMP0302 and IMP0405).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by I.D. Hume
Rights and permissions
About this article
Cite this article
Li, XS., Wang, DH. Seasonal adjustments in body mass and thermogenesis in Mongolian gerbils (Meriones unguiculatus): the roles of short photoperiod and cold. J Comp Physiol B 175, 593–600 (2005). https://doi.org/10.1007/s00360-005-0022-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-005-0022-2