Abstract
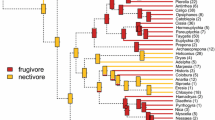
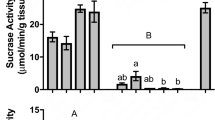
Flowerpiercers are the most specialized nectar-feeding passerines in the Neotropics. They are nectar robbers that feed on the sucrose-rich diet of hummingbirds. To test the hypothesis that flowerpiercers have converged with hummingbirds in digestive traits, we compared the activity of intestinal enzymes and the gut nominal area of cinnamon-bellied flowerpiercers (Diglossa baritula) with those of eleven hummingbird species. We measured sucrase, maltase, and aminopeptidase-N activities. To provide a comparative context, we also compared flowerpiercers and hummingbirds with 29 species of passerines. We analyzed enzyme activity using both standard allometric analyses and phylogenetically independent contrasts. Both approaches revealed the same patterns. With the exception of sucrase activity, hummingbirds’ digestive traits were indistinguishable from those of passerines. Sucrase activity was ten times higher in hummingbirds than in passerines. Hummingbirds and passerines also differed in the relationship between intestinal maltase and sucrase activities. Maltase activity was two times higher per unit of sucrase activity in passerines than in hummingbirds. The sucrase activity of D. baritula was much lower than that of hummingbirds, and not unlike that expected for a passerine of its body mass. With the exception of aminopeptidase-N activity, the digestive traits of D. baritula were not different from those of other passerines.




Similar content being viewed by others
Abbreviations
- K m :
-
Michaelis-Menten constant
- PDAP:
-
phenotypic diversity analysis program
- PIC:
-
phylogenetic independent contrast
- V max :
-
maximal reaction velocity
References
Afik D, Caviedes Vidal E, Martínez del Rio C, Karasov WH (1995) Dietary modulation of intestinal hydrolytic enzymes in yellow-rumped warblers. Am J Physiol 269:R413–R420
Albatshan HA, Sell JL, Piquer J, Mallarino E, Sotosalanova MF, Angel CR (1992) Responses of turkey poults to virginiamycin as influenced by litter condition and experimentally induced stunting syndrome. Poultry Sci 71:894–904
Alpers DH (1987) Digestion and absorption of carbohydrates and proteins. In: Johnson LR (ed) Physiology of the gastrointestinal tract. Raven Press, New York, pp 1469–1487
Baker HG (1977) Non-sugar chemical constituents of nectar. Apidologie 8:349–356
Baker HG, Baker I (1983a) A brief historical review of the chemistry of floral nectar. In: Bentley B, Ellias T (eds) The biology of nectaries. Columbia University Press, New York, pp 126–152
Baker HG, Baker I (1983b) Floral nectar constituents in relation to pollinator type. In: Jones CE, Little RJ (eds) Handbook of experimental pollination biology. Scientific & Academic Press, New York, pp 117–141
Bennett PM, Harvey PH (1987) Active and resting metabolism in birds—allometry, phylogeny and ecology. J Zool (Lond) 213:327–363
Beuchat CA, Chaplin SB, Morton ML (1979) Ambient temperature and the daily energetics of two species of hummingbirds, Calypte anna and Selasphorus rufus. Physiol Zool 52:280–295
Beuchat CA, Calder WA, Braun EJ (1990) The integration of osmoregulation and energy balance in hummingbirds. Physiol Zool 63:1059–108
Beuchat CA, Preest MR, Braun EJ (1999) Glomerular and medullary architecture in the kidney of Anna’s hummingbird. J Morphol 240:95–100
Biviano AB, Martínez del Rio C, Phillips DL (1993) Ontogeny of intestine morphology and intestinal disaccharidases in chickens (Gallus gallus) fed contrasting purified diets. J Comp Physiol B 163:508–518
Burns KJ (1997) Molecular systematics of tanagers (Thraupinae): evolution and biogeography of a diverse radiation of neotropical birds. Mol Phylogenet Evol 8:334–348
Calder WA (1984) Size, function and life history. Harvard University Press, Cambridge
Caviedes Vidal E, Afik D, Martínez del Rio C, Karasov WH (2000) Dietary modulation of intestinal enzymes of the house sparrow (Passer domesticus): testing an adaptative hypothesis. Comp Biochem Phys A 125:11–24
Felsenstein J (1985) Phylogenies and the comparative method. Am Nat 125:1–15
Ford HA (1985) Nectarivory and pollination by birds in southern Australia and Europe. Oikos 44:127–131
Frappell PB, Hinds DS, Boggs DF (2001) Scaling of respiratory variables and the breathing pattern in birds: an allometric and phylogenetic approach. Physiol Biochem Zool 74:75–89
Garland T Jr, Adolph SC (1994) Why not to do two-species comparative studies: limitations on inferring adaptation. Physiol Zool 67:797–828
Garland T Jr, Harvey PH, Ives AR (1992) Procedures for the analysis of comparative data using phylogenetically independent contrasts. Syst Biol 41:18–32
Garland T Jr, Dickerman AW, Janis CM, Jones JA (1993) Phylogenetic analysis of covariance by computer simulation. Syst Biol 42:265–292
Garland T Jr, Midford PE, Ives AR (1999) An introduction to phylogenetically based statistical methods, with a new method for confidence intervals on ancestral states. Am Zool 39:374–388
Hu CB, Spiess M, Semenza G (1987) The mode of anchoring and precursor forms of sucrase-isomaltase and maltase-glucoamylase in chicken intestinal brush-border membrane: phylogenetic implications. Biochim Biophys Acta 896:275–286
Isler ML, Isler PR (1999) The Tanagers. Natural history, distribution and identification. Smithsonian Institution Press, Washington, DC
Jackson S, Diamond J (1995) Ontogenic development of gut function, growth, and metabolism in a wild bird, the red jungle fowl. Am J Physiol 38:R1163–R1173
Kania RK, Santiago NA, Gray GM (1977) Intestinal surface amino-oligopeptidases. II. Substrate kinetics and topography of the active site. J Biol Chem 252:4929–4934
Karasov WH, Phan D, Diamond JM, Carpenter FL (1986) Food passage and intestinal nutrient absorption in hummingbirds. Auk 103:453–464
Levey DJ, Place AR, Rey PJ, Martínez del Rio C (1999) An experimental test of dietary enzyme modulation in pine warblers Dendroica pinus. Physiol Biochem Zool 72:576–587
Lotz C, Martínez del Rio C (2004) The ability of rufous hummingbirds (Selasphorus rufus) to dilute and concentrate excreta. J Avian Biol (in press)
Maroux, S, Louvard D, Baratti J (1973) The aminopeptidase from hog intestinal brush-border. Biochim Biophys Acta 321:282–295
Martínez del Rio C (1990) Dietary, phylogenetic, and ecological correlates of intestinal sucrase and maltase activity in birds. Physiol Zool 63:987–1011
Martínez del Rio C (1994) Nutritional ecology of fruit-eating and flower-visiting birds and bats. In: Chivers D, Langer P (eds) The digestive system: food, form and function. Cambridge University Press, Cambridge, pp 103–127
Martínez del Rio C, Stevens BR (1989) Physiological constraint on feeding behavior: intestinal membrane disaccharidases of the starling. Science 243:794–796
Martínez del Rio C, Brugger KE, Rios JL, Vergara ME, Witmer M (1995) An experimental and comparative study of dietary modulation of intestinal enzymes in European starlings (Sturnus vulgaris). Physiol Zool 68:490–511
Martínez del Rio C, Schondube JE, McWhorter TJ, Herrera LG (2001) Intake responses in nectar feeding birds: digestive and metabolic causes, osmoregulatory consequences, and coevolutionary effects. Am Zool 41:902–915
McWhorter TJ, López-Calleja MV (2000) The integration of diet, physiology, and ecology of nectar-feeding birds. Rev Chil Hist Nat 73:451–460
McWhorter TJ, Martínez del Rio C (1999) Food ingestion and water turnover in hummingbirds: how much dietary water is absorbed? J Exp Biol 202:2851–2858
McWhorter TJ, Martínez del Rio C (2000) Does gut function limit hummingbird food intake? Physiol Biochem Zool 73:313–324
McWhorter TJ, Martínez del Rio C, Pinshow B (2003) Modulation of ingested water absorption by Palestine sunbirds: evidence for adaptive regulation. J Exp Biol 206:659–666
McWhorter TJ, Powers DR, Matinez del Rio C (2004) Are hummingbirds facultatively ammoniotelic? Nitrogen excretion and requirements as a function of body size. Physiol Biochem Zool (in press)
McWilliams SR, Caviedes Vidal E, Karasov WH (1999) Digestive adjustments in cedar waxwings to high feeding rate. J Exp Zool 283:394–407
Meynard C, López-Calleja MV, Bozinovic F (1999) Digestive enzymes of a small avian herbivore, the rufous-tailed plantcutter. Condor 101:904–907
Nagy KA, Girard IA, Brown TK (1999) Energetics of free-ranging mammals, reptiles, and birds. Annu Rev Nutr 19:247–277
Powers DR, Conley TM (1994) Field metabolic-rate and food-consumption of 2 sympatric hummingbird species in southeastern Arizona. Condor 96:141–150
Ramsey FL, Schafer DW (1996) The statistical sleuth: a course in methods of data analysis. Duxbury Press, Albany, NY
Reynolds PS, Lee RM (1996) Phylogentic analysis of avian energetics: passerines and nonpasserines do not differ. Am Nat 147:735–759
Sabat P (2000) Intestinal disaccharidases and aminopeptidase-N in two species of Cinclodes (Passerine: Furnaridae). Rev Chil Hist Nat 73:345–350
Sabat P, Novoa F, Bozinovic F, Martínez del Rio C (1998) Dietary flexibility and intestinal plasticity in birds: A field and laboratory study. Physiol Zool 71:226–236
Schondube JE (2003) Flowerpiercers and hummingbirds: a comparative study of nectar feeding strategies in birds. PhD thesis, University of Arizona, Tucson
Schondube JE, Martinez del Rio C (2003a) The flowerpiercer’s hook: an experimental test of an evolutionary trade-off. Proc R Soc London Ser B 270:195–198
Schondube JE, Martinez del Rio C (2003b) Concentration-dependent sugar preferences in nectar-feeding birds: mechanisms and consequences. Funct Ecol 17:445–453
Schondube JE, Herrera-M LG, Martínez del Rio C (2001) Diet and the evolution of digestion and renal function in phyllostomid bats. Zool Anal Complex Syst 104:59–73
Sell JL, Koldovsky O, Reid. BL (1988) Intestinal disaccharidases of young turkeys: temporal development and influence of diet composition. Poultry Sci 68:265–277
Semenza G, Corcelli A (1986) The absorption of sugars and amino acids across the small intestine. In: Desnuelle P, Sjöstrom H, Norén A (eds) Molecular and cellular basis of digestion. Elsevier, New York, pp 381–412
Sibley CG, Ahlquist JE (1990) Phylogeny and classification of birds. Yale University Press, New Haven
Skutch AF (1954) Life histories of Central American birds. Pacific Coast avifauna, vol 31. Cooper Onithological Society, Berkeley
Sørensen H, Norén O, Danielsen M (1982) Amphiphilic pig intestinal microvillus lactase/glucoamylase, structure and specificity. Eur J Biochem 126:559–568
Stiles FG (1981) Geographical aspects of bird-flower coevolution, with particular reference to Central America. Ann Mo Bot Gard 68:323–351
Stiles FG (1985) Seasonal patterns and coevolution in the hummingbird-flower community of a Costa Rican subtropical forest. Ornithol Monogr 36:757–787
Suarez RK, Gass CL (2002) Hummingbird foraging and the relation between bioenergetics and behaviour. Comp Biochem Physiol A 133:335–343
Tiebout HM (1991) Daytime energy management by tropical hummingbirds: responses to foraging constraint. Ecology 72:839–851
Uni Z, Platin R, Sklan D (1998) Cell proliferation in chicken intestinal epithelium occurs both in the crypt and along the villus. J Comp Physiol B 168:241–247
Vuilleumier F (1969) Systematics and evolution in Diglossa (Aves: Coerebidae). Am Mus Novit 2381:1–44
Weathers WW, Stiles FG (1989) Energetics and water balance in free-living tropical hummingbirds. Condor 91:324–331
West GB, Brown JH, Enquist BJ (1997) A general model for the origin of allometric scaling laws in biology. Science 276:122–126
West GB, Brown JH, Enquist BJ (1999) The fourth dimension of life: fractal geometry and allometric scaling of organisms. Science 284:1677–1679
Witmer MC, Martínez del Rio C (2001) The membrane-bound intestinal enzymes of waxwings and thrushes: adaptive and functional implications of patterns of enzyme activity. Physiol Biochem Zool 74:584–593
Wolf LL, Hainsworth FR, Gill FB (1975) Foraging efficiencies and time budgets in nectar-feeding birds. Ecology 56:117–128
Acknowledgements
Irma Ruan-Tejeda, David Valenzuela, Jose G. Carrillo, Claudet Guerrero and Mario López-Vieyra helped during field-work. Melissa K. Schroer and Enriquena Bustamante helped with the enzyme assays. Todd McWhorter shared unpublished enzyme activity for several bird species. Claudia Nepote provided critical logistical support during data analysis. Francisco Ornelas kindly provided us with unpublished data on hummingbird phylogenetic relationships. Kevin Bonine offered his time and expertise to generate the values for the phylogenetic independent contrasts. Funding for this project came from the Department of Ecology and Evolutionary Biology, University of Arizona, the Chonadobe Fund for Third World Students, and a doctoral fellowship from CONACyT, Mexico (125663). This research project was conducted with permission from the National Institute of Ecology (INE), Mexico, and approved by the University of Arizona Institutional Animal Care and Use Committee.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Heldmaier
Rights and permissions
About this article
Cite this article
Schondube, J.E., Martinez del Rio, C. Sugar and protein digestion in flowerpiercers and hummingbirds: a comparative test of adaptive convergence. J Comp Physiol B 174, 263–273 (2004). https://doi.org/10.1007/s00360-003-0411-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-003-0411-3