Abstract
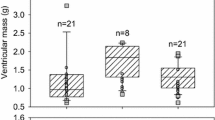
Ca2+-induced Ca2+ release (CICR) mechanism of cardiac excitation–contraction (e–c) coupling is dependent on the close apposition between the sarcolemmal dihydropyridine receptors (DHPR) and the sarcoplasmic reticulum (SR) ryanodine receptors (RyR). In particular, high RyR/DHPR ratio is considered to reflect strong dependence on SR Ca2+ stores for the intracellular Ca2+ transient. To indirectly evaluate the significance of CICR in fish hearts, densities of cardiac DHPRs and RyRs were compared in ventricular homogenates of three fish species (burbot, rainbow trout, and crucian carp) and adult rat by [3H] PN200–110 and [3H] ryanodine binding. The density of RyRs was significantly (P<0.05) higher in the adult rat (124±10 channels/μm3 myocyte volume) than in any of the fish species. Among the fish species, cold-acclimated (4 °C) trout had more RyRs than burbot, and crucian carp. The density of DHPRs was highest in the trout heart. RyR/DHPR ratio was significantly (P<0.05) higher in rat (4.1±0.5) than in the fish hearts (varying from 0.97±0.16 to 1.91±0.49) suggesting that "mammalian type" CICR is less important during e–c coupling in fish ventricular myocytes. In rainbow trout, acclimation to cold did not affect the RyR/DHPR ratio, while in crucian carp it was depressed in cold-acclimated animals (4 °C; 0.97±0.16) when compared to warm-acclimated fish (23 °C; 1.91±0.49). Although RyR/DHPR ratios were relatively low in fish hearts, there was a close correlation (r 2=0.78) between the RyR/DHPR ratio and the magnitude of the Ry-sensitive component of contraction in ventricular muscle among the fish species examined in this study.




Similar content being viewed by others
Abbreviations
- AP :
-
action potential
- B max :
-
number of DHPR or RyR sites (pmol mg−1)
- CA :
-
cold-acclimated
- CICR :
-
Ca2+-induced Ca2+ release
- DHPR :
-
dihydropyridine receptor
- ICa:
-
Ca2+ current
- Kd :
-
affinity of DHPR or RyR sites (nmol l−1)
- PMSF :
-
phenylmethylsulfonyl fluoride
- RyR :
-
ryanodine receptor
- Ry :
-
ryanodine
- SL :
-
sarcolemma
- SR :
-
sarcoplasmic reticulum
- WA :
-
warm-acclimated
References
Aho E, Vornanen M (1998) Ca2+-ATPase activity and Ca2+ uptake by sarcoplasmic reticulum in fish heart: effects of thermal acclimation. J Exp Biol 201:525–532
Aho E, Vornanen M (1999) Contractile properties of atrial and ventricular myocardium of the heart of rainbow trout (Oncorhynchus mykiss): effects of thermal acclimation. J Exp Biol 202:2663–2677
Bers DM (2002) Cardiac excitation-contraction coupling. Nature 415:198–205
Bers DM, Stiffel VA (1993) Ratio of ryanodine to dihydropyridine receptors in cardiac and skeletal muscle and implications for E–C-coupling. Am J Physiol 264:C1587–C1593
Bowler K, Tirri R (1990) Temperature dependence of the heart isolated from the cold or warm acclimated perch (Perca fluviatilis). Comp Biochem Physiol A 96:177–180
Chugun A, Oyamada T, Temma K, Hara Y, Kondo H (1999) Intracellular Ca2+ storage sites in the carp heart: comparison with the rat heart. Comp Biochem Physiol A 123:61–67
Conover WJ (1980) Practical nonparametric statistics. John Wiley, New York, pp 228–237
Fabiato A (1983) Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol 245:C1–C14
Fabiato A, Fabiato F (1978) Calcium-induced release of calcium from the sarcoplasmic reticulum of skinned cells from adult human, dog, cat, rabbit, rat, and frog hearts and from fetal and new-born rat ventricles. Ann N Y Acad Sci 307:491–522
Farrell AP, Milligan CL (1986) Myocardial intracellular pH in a perfused rainbow trout heart during extracellular acidosis in the presence and absence of adrenaline. J Exp Biol 125:347–359
Frank JS, Langer GA (1974) The myocardial interstitium: its structure and its role in ionic exchange. J Cell Biol 60:586–601
Harwood CL, Howarth C, Altringham JD, White E (2000). Rate-dependent changes in cell shortening, intracellular Ca2+ levels and membrane potential in single, isolated rainbow trout (Oncorhynchus mykiss) ventricular myocytes. J Exp Biol 203:493–504
Hove-Madsen L (1992) The influence of temperature on ryanodine sensitivity and the force-frequency relationship in the myocardium of rainbow trout. J Exp Biol 167:47–60
Hove-Madsen L, Llach A, Tort L (2000) Na+/Ca2+-exchange activity regulates contraction and SR Ca2+ content in rainbow trout atrial myocytes. Pflügers Arch 438:545–552
Katsube Y, Yokoshiki H, Nguyen L, Sperelakis N (1996) Differences in isoproterenol stimulation of Ca2+ current of rat ventricular myocytes in neonatal compared to adult. Eur J Pharmacol 317:391–400
Keen JE, Farrell AP, Tibbits GF, Brill RW (1992) Cardiac physiology in tunas. II. Effect of ryanodine, calcium and adrenaline on force-frequency relationships in atrial strips from skipjack tuna, Katsuwonus pelamis. Can J Zool 70:1211–1217
Keen JE, Vianzon D-M, Farrell AP, Tibbits GF (1994) Effect of temperature and temperature acclimation on the ryanodine sensitivity of the trout myocardium. J Comp Physiol B 164:438–443
Kim CS, Coyne MD, Gwathmey JK (2000) Voltage-dependent calcium channels in ventricular cells of rainbow trout: effect of temperature changes in vitro. Am J Physiol 278:R524–R534
Lai FA, Erickson H, Block BA, Meissner G (1987) Evidence for a junctional feet-ryanodine receptor complex from sarcoplasmic reticulum. Biochem Biophys Res Commun 143:704–709
Lowry OH, Rosenborough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Mendez J, Keys A (1960) Density and composition of mammalian muscle. Metabolism 9:184–188
Mercier C, Axelsson M, Imbert N, Claireaux G, Lefrançois C, Altimiras J, Farrell AP (2002) In vitro cardiac performance in triploid brown trout at two acclimation temperatures. J Fish Biol 60, 117–133
Milnes JT, MacLeod KT (2000) Reduced ryanodine receptor to dihydropyridine receptor ratio may underlie slowed contraction in a rabbit model of left ventricular cardiac hypertrophy. J Mol Cell Cardiol 33:473–485
Otsu K, Willard HF, Khanna VK, Zorzati F, Green NM, MacLennan DH (1990) Molecular cloning of cDNA encoding the Ca2+ release channel (ryanodine receptor) of rabbit cardiac muscle sarcoplasmic reticulum. J Biol Chem 265:13472–13483
Puglisi JL, Yuan W, Bassani JWM, Bers DM (1999) Ca2+ influx through Ca2+ channels in rabbit ventricular myocytes during action potential clamp. Influence of temperature. Circ Res 85:E7–E16
Rantin RT, Gesser H, Kalin AL, Guerra CDR, De Freitas JC, Driedzic WR (1998) Heart performance, Ca2+ regulation and energy metabolism at high temperatures in the Bathybobius soporator, a tropical marine teleost. J Therm Biol 23:31–39
Reuter, H (1983) Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature 301:569–574
Santer RM, Cobb JLS (1972) The fine structure of the heart of the teleost, Pleuronectes platessa L. Z Zellforsch 131:1–14
Satoh H, Delbridge LMD, Blatter LA, Bers DM (1996) Surface:volume relationship in cardiac myocytes studied with confocal microscopy and membrane capacitance measurements: species-dependence and developmental effects. Biophys J 70:1494–1504
Scamps F, Mayoux E, Charlemagne D, Vassort G (1990) Calcium current in single cells isolated from normal and hypertrophied rat heart. Effects of beta-adrenergic stimulation. Circ Res 67:199–208
Shiels HA, Farrell AP (1997) The effects of temperature and adrenaline on the relative importance of sarcoplasmic reticulum in contributing calcium to force development in isolated ventricular trabeculae from rainbow trout. J Exp Biol 200:1607–1621
Shiels HA, Farrell AP (2000) The effect of ryanodine on isometric tension development in isolated ventricular trabeculae from Pacific mackerel (Scomber japonicus). Comp Biochem Physiol A 125:331–341
Shiels HA, Freud EV, Farrell AP (1999) The sarcoplasmic reticulum plays major role in isometric contraction in atrial muscle of yellowfin tuna. J Exp Biol 202:881–890
Sperelakis N, Katsube Y, Yokoshiki H, Sada H, Sumii K (1996) Regulation of the slow Ca2+ channels of myocardial cells. Mol Cell Biochem 163–164:85–98
Thomas MJ, Hamman BN, Tibbits GF (1996) Dihydropyridine and ryanodine binding in ventricles from rat, trout, dogfish and hagfish. J Exp Biol 199:1999–2009
Tibbits GF, Hove-Madsen L, Bers DM (1991) Calcium transport and the regulation of cardiac contractility in teleosts: a comparison with higher vertebrates. Can J Zool 69:2014–2019
Tiitu V, Vornanen M (2001) Cold acclimation suppresses the contractility of both atrial and ventricular muscle of the crucian carp (Carassius carassius L.) heart. J Fish Biol 59:141–156
Tiitu V, Vornanen M (2002) Regulation of cardiac contractility in a cold stenothermal fish, the burbot Lota lota L. J Exp Biol 205:1597–1606
Tiitu V, Vornanen M (2003) Does different thyroid state effect on the contractility of the cardiac muscle of eurythermal fish species, rainbow trout (Oncorhynchus mykiss, Walbaum)? J Therm Biol 28:35–42
Vornanen M (1989) Regulation of contractility of the fish (Carassius carassius L.) heart ventricle. Comp Biochem Physiol C 94:477–483
Vornanen M (1996) Effects of extracellular calcium on the contractility of warm-and cold-acclimated crucian carp heart. J Comp Physiol B 166:1–11
Vornanen M (1997) Sarcolemmal Ca2+ influx through L-type Ca2+ channels in ventricular myocytes of a teleost fish. Am J Physiol 272: R1432–R1440
Vornanen M (1998) L-type Ca2+ current in fish cardiac myocytes: effects of thermal acclimation and β-adrenergic stimulation. J Exp Biol 201:533–547
Vornanen M, Shiels HA, Farrell AP (2002) Plasticity of excitation-contraction coupling in fish cardiac myocytes. Comp Biochem Physiol A 132:827–846
Wang S-Q, Song L-S, Lakatta EG, Cheng H (2001) Ca2+ signalling between single L-type Ca2+ channels and ryanodine receptors in heart cells. Nature 410:592–596
Acknowledgements
This work was supported by the Academy of Finland (Project Nos. 63090 and 78045). We would like to thank the Kontiolahti fish farm for donating the trout. In addition, we would like to thank local fisherman, Terho Laitinen, for supplying the burbot and Anita Kervinen for technical assistance in binding studies. The experiments comply with the current legislation for animal protection in Finland.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Heldmaier
Rights and permissions
About this article
Cite this article
Tiitu, V., Vornanen, M. Ryanodine and dihydropyridine receptor binding in ventricular cardiac muscle of fish with different temperature preferences. J Comp Physiol B 173, 285–291 (2003). https://doi.org/10.1007/s00360-003-0334-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-003-0334-z