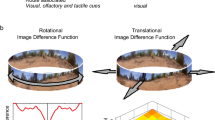
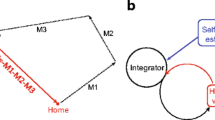
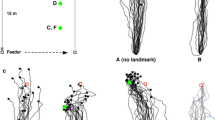
Abstract
Rüdiger Wehner’s work on insect orientation and navigation has influenced many scientists studying navigation, not only in ants and bees, but in other animals as well. We review the scientific legacy of six topics arising from Wehner’s work on navigation. The polarisation compass is a chapter with a lot of behavioural and neurobiological detail. It has influenced the study of polarisation vision in other systems, and led Wehner to formulate the concept of a matched filter. The matched filter has probably had earlier formulations, but Wehner’s paper on it has been much cited in studies on navigation and in other fields. The polarisation compass serves the task of path integration in insects. Work on path integration took off in the 1980s with work on desert ants and rodents. The use of terrestrial visual cues, landmarks or the panorama in view-based matching is another major theme of navigational research today. Search strategies were also well described in desert ants, and this line of research helped to launch theoretical and empirical developments in searching behaviour, now a lively area of research. Finally, robotic work has often drawn inspiration from work on insect navigation. We end with some discussion of current research directions.




Similar content being viewed by others
References
Abbott A, Callaway E (2014) Prize for place cells: discoverers of brain’s navigation system get physiology Nobel. Nature 514:153
Andel D, Wehner R (2004) Path integration in desert ants, Cataglyphis fortis: how to make a homing ant run away from home. Proc R Soc B 271:1485–1489
Avni R, Eilam D (2008) On the border: perimeter patrolling as a transitional exploratory phase in a diurnal rodent, the fat sand rat (Psammomys obesus). Anim Cogn 11:311–318
Avni R, Zadicario P, Eilam D (2006) Exploration in a dark open field: a shift from directional to positional progression and a proposed model of acquiring spatial information. Behav Brain Res 171:313–323
Backhaus W (1991) Color opponent coding in the visual system of the honeybee. Vision Res 31:1381–1397
Baddeley B, Graham P, Philippides A, Husbands P (2011) Holistic visual encoding of ant-like routes: navigation without waypoints. Adapt Behav 19:3–15
Baddeley B, Graham P, Husbands P, Philippides A (2012) A model of ant route navigation driven by scene familiarity. PLoS Comput Biol 8:e1002336
Barlow HB (1953) Summation and inhibition in the frog’s retina. J Physiol 119:69–88
Bech M, Homberg U, Pfeiffer K (2014) Receptive fields of locust brain neurons are matched to polarization patterns of the sky. Curr Biol 24:2124–2129
Becker L (1958) Untersuchungen über das Heimfindevermögen der Bienen. Z Vergl Physiol 41:1–25
Benhamou S (2007) How many animals really do the Lévy walk? Ecol 88:1962–1969
Bennett ATD (1993) Spatial memory in a food storing corvid. I. Near tall landmarks are primarily used. J Comp Physiol A 173:193–207
Bernard GD, Wehner R (1977) Functional similarities between polarization vision and color vision. Vision Res 17:1019–1028
Beugnon G, Lachaud J-P, Chagné P (2005) Use of long-term stored vector information in the neotropical ant Gigantiops destructor. J Insect Behav 18:415–432
Buchanan M (2008) The mathematical mirror to animal nature. Nature 453:714–716
Buehlmann C, Hansson BS, Knaden M (2012) Desert ants learn vibration and magnetic landmarks. PLoS One 7:e33117
Bühlmann C, Cheng K, Wehner R (2011) Vector-based and landmark-guided navigation in desert ants inhabiting landmark-free and landmark-rich environments. J Exp Biol 214:2845–2853
Burns LD (2013) A vision of our transport future. Nature 497:181–182
Capaldi EA, Smith AD, Osborne JL, Fahrbach SE, Farris SM, Reynolds DR, Edwards AS, Martin A, Robinson GE, Poppy GM, Riley JR (2000) Ontogeny of orientation flight in the honeybee revealed by harmonic radar. Nature 403:537–540
Cartwright BA, Collett TS (1979) How honeybees know their distance from a near-by visual landmark. J Exp Biol 82:367–372
Cartwright BA, Collett TS (1982) How honey bees use landmarks to guide their return to a food source. Nature 295:560–564
Cartwright BA, Collett TS (1983) Landmark learning in bees. J Comp Physiol A 151:521–543
Cheng K (2012a) Arthropod navigation: ants, bees, crabs, spiders finding their way. In: Zentall TR, Wasserman EA (eds) The Oxford handbook of comparative cognition. Oxford University Press, Oxford, pp 347–365
Cheng K (2012b) How to navigate without maps: the power of taxon-like navigation in ants. Comp Cogn Behav Rev 7:1–22
Cheng K, Spetch ML (1998) Mechanisms of landmark use in mammals and birds. In: Healy S (ed) Spatial representation in animals. Oxford University Press, Oxford, pp 1–17
Cheng K, Collett TS, Wehner R (1986) Honeybees learn the colours of landmarks. J Comp Physiol A 159:69–73
Cheng K, Collett TS, Pickhard A, Wehner R (1987) The use of visual landmarks by honeybees: bees weight landmarks according to their distance from the goal. J Comp Physiol A 161:469–475
Cheng K, Spetch ML, Kelly DM, Bingman VP (2006) Small-scale spatial cognition in pigeons. Behav Processes 72:115–127
Cheng K, Shettleworth SJ, Huttenlocher J, Rieser JJ (2007) Bayesian integration of spatial information. Psychol Bull 133:625–637
Cheng K, Narendra A, Sommer S, Wehner R (2009) Traveling in clutter: navigation in the Central Australian desert ant Melophorus bagoti. Behav Processes 80:261–268
Cheng K, Middleton EJT, Wehner R (2012) Vector-based and landmark-guided navigation in desert ants of the same species inhabiting landmark-free and landmark-rich environments. J Exp Biol 215:3169–3174
Cheng K, Schultheiss P, Schwarz S, Wystrach A, Wehner R (2014) Beginnings of a synthetic approach to desert ant navigation. Behav Processes 102:51–61
Cheung A, Hiby L, Narendra A (2012) Ant navigation: fractional use of the home vector. PLoS One 7:e50451
Chittka L, Kunze J, Shipman C, Buchmann SL (1995) The significance of landmarks for path integration in homing honeybee foragers. Naturwissenschaften 82:341–343
Christian KA, Morton SR (1992) Extreme thermophilia in a Central Australian ant, Melophorus bagoti. Physiol Zool 65:885–905
Collett M (2010) How desert ants use a visual landmark for guidance along a habitual route. Proc Natl Acad Sci USA 107:11638–11643
Collett M (2012) How navigational guidance systems are combined in a desert ant. Curr Biol 22:927–932
Collett TS, Collett M (2000) Path integration in insects. Curr Opin Neurobiol 10:757–762
Collett TS, Land MF (1975) Visual spatial memory in a hoverfly. J Comp Physiol 100:59–84
Collett TS, Fry S, Wehner R (1993) Sequence learning by honeybees. J Comp Physiol A 172:693–706
Collett M, Collett TS, Bisch S, Wehner R (1998) Local and global vectors in desert ant navigation. Nature 394:269–272
Collett M, Chittka L, Collett TS (2013) Spatial memory in insect navigation. Curr Biol 23:R789–R800
Dacke M, Nilsson DE, Scholtz CH, Byrne M, Warrant EJ (2003) Insect orientation to polarized moonlight. Nature 424:33
Dacke M, Byrne MJ, Scholtz CH, Warrant EJ (2004) Lunar orientation in a beetle. Proc R Soc B 271:361–365
Dacke M, Baird E, Byrne M, Scholtz CH, Warrant EJ (2013) Dung beetles use the Milky Way for orientation. Curr Biol 23:298–300
Darwin C (1873) Origin of certain instincts. Nature 7:417–418
de Jager M, Weissing FJ, Herman PMJ, Nolet BA, van de Koppel J (2011) Lévy walks evolve through interaction between movement and environmental complexity. Science 332:1551–1553
de Jager M, Weissing FJ, Herman PMJ, Nolet BA, van de Koppel J (2012) Response to comment on “Lévy walks evolve through interaction between movement and environmental complexity”. Science 335:918-d
Dukas R (2008) Evolutionary biology of insect learning. Annu Rev Entomol 53:145–160
Dyer FC (1987) Memory and sun compensation by honey bees. J Comp Physiol A 160:621–633
Dyer F (1996) Spatial memory and navigation by honeybees on the scale of the foraging range. J Exp Biol 199:147–154
Edwards AM (2011) Overturning conclusions of Lévy flight movement patterns by fishing boats and foraging animals. Ecol 92:1247–1257
Edwards AM, Phillips RA, Watkins NW, Freeman MP, Murphy EJ, Afanasyev V, Buldyrev SV, da Luz MGE, Raposo EP, Stanley EH, Viswanathan GM (2007) Revisiting Lévy flight search patterns of wandering albatrosses, bumblebees and deer. Nature 449:1044–1048
el Jundi B, Pfeiffer K, Heinze S, Homberg U (2014) Integration of polarization and chromatic cues in the insect sky compass. J Comp Physiol A 200:575–589
Esch HE, Burns JE (1995) Honeybees use optic flow to measure the distance of a food source. Naturwissenschaften 82:38–40
Esch HE, Zhang S, Srinivasan MV, Tautz J (2001) Honeybee dances communicate distances measured by optic flow. Nature 411:581–583
Etienne A (1980) The orientation of the Golden hamster to its nest-site after the elimination of various sensory cues. Experientia 36:1048–1050
Etienne AS, Jeffery KJ (2004) Path integration in mammals. Hippocampus 14:180–192
Etienne AS, Maurer R, Séguinot V (1996) Path integration in mammals and its interaction with visual landmarks. J Exp Biol 199:201–209
Franz MO, Mallot HA (2000) Biomimetic robot navigation. Robot Auton Syst 30:133–153
Franz MO, Scholkopf B, Mallot HA, Bülthoff HH (1998) Learning view graphs for robot navigation. Auton Robot 5:111–125
Fyhn M, Molden S, Witter MP, Moser EI, Moser M-B (2004) Spatial representation in the entorhinal cortex. Science 305:1258–1264
Gallistel CR (1990) The organization of learning. MIT Press, Cambridge
Gibson B, Wilks T (2008) The use of self-motion cues and landmarks by Clark’s nutcrackers (Nucifraga columbiana) during a small-scale search task. Anim Behav 76:1305–1317
Glasauer S, Amorim MA, Vitte E, Berthoz A (1994) Goal-directed linear locomotion in normal and labyrinthine-defective subjects. Exp Brain Res 98:323–335
Goddard SM, Forward RB (1991) The role of underwater polarized-light pattern, in sun compass navigation of the grass shrimp, Palaemonetes vulgaris. J Comp Physiol A 169:479–491
Goodale MA, Milner AD (1992) Separate visual pathways for perception and action. Trends Neurosci 15:20–25
Görner P (1958) Die optische und kinästhetische Orientierung der Trichterspinne Agelena labyrinthica (Cl.). Z Vergleich Physiol 41:111–153
Gould-Beierle K, Kamil AC (1998) Use of landmarks in three species of food-storing corvids. Ethol 104:361–378
Graham P, Cheng K (2009a) Ants use the panoramic skyline as a visual cue during navigation. Curr Biol 19:R935–R937
Graham P, Cheng K (2009b) Which portion of the natural panorama is used for view based navigation in the Australian desert ant? J Comp Physiol A 195:681–689
Gross CG (2002) Genealogy of the “grandmother cell”. Neuroscientist 8:512–518
Haferlach T, Wessnitzer J, Mangan M, Webb B (2007) Evolving a neural model of insect path integration. Adapt Behav 15:273–287
Hafting T, Fyhn M, Molden S, Moser M-B, Moser EI (2005) Microstructure of a spatial map in the entorhinal cortex. Nature 436:801–806
Hartmann G, Wehner R (1995) The ant’s path integration system: a neural architecture. Biol Cybern 73:483–497
Hawryshyn CW (1992) Polarization vision in fish. Am Sci 80:164–175
Helbig AJ (1990) Depolarization of natural skylight disrupts orientation of an avian nocturnal migrant. Experientia 46:755–758
Hemmi JM, Zeil J (2003a) Burrow surveillance in fiddler crabs-I: description of behaviour. J Exp Biol 206:3935–3950
Hemmi JM, Zeil J (2003b) Burrow surveillance in fiddler crabs-II: the sensory cues. J Exp Biol 206:3951–3961
Hemmi JM, Zeil J (2003c) Robust judgement of inter-object distance by an arthropod. Nature 421:160–163
Hills TT (2006) Animal foraging and the evolution of goal-directed cognition. Cogn Sci 30:3–41
Hills TT (2011) The evolutionary origins of cognitive control. Topics Cogn Sci 3:231–237
Hoffmann G (1983a) The random elements in the systematic search behavior of the desert isopod Hemilepistus reaumuri. Behav Ecol Sociobiol 13:81–92
Hoffmann G (1983b) The search behavior of the desert isopod Hemilepistus reaumuri as compared with a systematic search. Behav Ecol Sociobiol 13:93–106
Hoffmann G (1985a) The influence of landmarks on the systematic search behavior of the desert isopod Hemilepistus reaumuri I: role of the landmark made by the animal. Behav Ecol Sociobiol 17:325–334
Hoffmann G (1985b) The influence of landmarks on the systematic search behavior of the desert isopod Hemilepistus reaumuri II problems with similar landmarks and their solution. Behav Ecol Sociobiol 17:335–348
Hurvich LM, Jameson D (1957) An opponent-process theory of color vision. Psychol Rev 64:384–404
Jacob F (1977) Evolution and tinkering. Science 196:1161–1166
Jakobi N (1997) Evolutionary robotics and the radical envelope-of-noise hypothesis. Adapt Behav 6:325–368
Jansen VAA, Mashanova A, Petrovskii S (2012) Comment on “Lévy walks evolve through interaction between movement and environmental complexity”. Science 335:918-c
Kamil AC, Jones JE (1997) Clark’s nutcrackers learn geometric relationships among landmarks. Nature 390:276–279
Knaden M, Wehner R (2005) Nest mark orientation in desert ants Cataglyphis: what does it do to the path integrator? Anim Behav 70:1349–1354
Knaden M, Tinaut A, Cerda X, Wehner S, Wehner R (2005) Phylogeny of three parapatric species of desert ants, Cataglyphis bicolor, C. viatica, and C. savignyi: a comparison of mitochondrial DNA, nuclear DNA, and morphological data. Zool 108:167–177
Knaden M, Tinaut A, Stökl J, Wehner R (2012) Molecular phylogeny of the desert ant genus Cataglyphis (Hymenoptera: Formicidae). Myrmecol News 16:123–132
Kohler M, Wehner R (2005) Idiosyncratic route memories in desert ants, Melophorus bagoti: how do they interact with path integration vectors? Neurobiol Learn Mem 83:1–12
Labhart T (1988) Polarization-opponent interneurons in the insect visual system. Nature 331:435–437
Labhart T (1996) How polarization-sensitive interneurones of crickets perform at low degrees of polarization. J Exp Biol 199:1467–1475
Lambrinos D, Maris M, Kobayashi H, Labhart T, Pfeifer R, Wehner R (1997) An autonomous agent navigating with a polarized light compass. Adapt Behav 6:131–161
Lambrinos D, Möller R, Labhart T, Pfeifer R, Wehner R (2000) A mobile robot employing insect strategies for navigation. Robot Auton Syst 30:39–64
Lebhardt F, Ronacher B (2014) Interactions of the polarization and the sun compass in path integration of desert ants. J Comp Physiol A 200:711–720
Legge ELG, Spetch ML, Cheng K (2010) Not using the obvious: desert ants, Melophorus bagoti, learn local vectors but not beacons in an arena. Anim Cogn 13:849–860
Lent D, Graham P, Collett TS (2013) Visual scene perception in navigating wood ants. Curr Biol 23:684–690
Lettvin JY, Maturana HR, McCulloch WS, Pitts WH (1959) What the frog’s eye tells the frog’s brain. Proc Inst Radio Engin 47:1940–1951
Loomis JM, Klatzky RL, Golledge RG, Cicinelli JG, Pellegrino JW, Fry PA (1993) Nonvisual navigation by blind and sighted: assessment of path integration ability. J Exp Psychol Gen 122:73–91
Lunau K (2000) The ecology and evolution of visual pollen signals. Plant Syst Evol 222:89–111
Maaswinkel H, Whishaw IQ (1999) Homing with locale, taxon, and dead reckoning strategies by foraging rats: sensory hierarchy in spatial navigation. Behav Brain Res 99:143–152
Margolis E, Laurence S (2013) In defense of nativism. Phil Stud 165:693–718
Mataric MJ (1995) Designing and understanding adaptive group behavior. Adapt Behav 4:51–80
Mather J (1991) Navigation by spatial memory and use of visual landmarks in octopuses. J Comp Physiol A 168:491–497
McNaughton BL, Battaglia FP, Jensen O, Moser EI, Moser M-B (2006) Path integration and the neural basis of the ‘cognitive map’. Nature Rev Neurosci 7:663–678
Milner AD, Goodale MA (1995) The visual brain in action. Oxford University Press, New York
Mittelstaedt M-L, Mittelstaedt H (1980) Homing by path integration in a mammal. Naturwissenschaften 67:566
Möller R (2001) Do insects use templates or parameters for landmark navigation? J Theor Biol 210:33–45
Möller R (2002) Insects could exploit UV-green contrast for landmark navigation. J Theor Biol 214:619–631
Moller P, Görner P (1994) Homing by path integration in the spider Agelena labyrinthica Clerck. J Comp Physiol A 174:221–229
Möller R, Lambrinos D, Pfeifer R, Labhart T, Wehner R (1998) Modeling ant navigation with an autonomous agent. In: Pfeifer R, Blumberg B, Meyer J-A, Wilson SW (eds) From animals to animats. MIT Press, Cambridge, pp 185–194
Müller M, Wehner R (1988) Path integration in desert ants, Cataglyphis fortis. Proc Natl Acad Sci USA 85:5287–5290
Müller M, Wehner R (1994) The hidden spiral: systematic search and path integration in desert ants, Cataglyphis fortis. J Comp Physiol A 175:525–530
Müller M, Wehner R (2010) Path integration provides a scaffold for landmark learning in desert ants. Curr Biol 20:1368–1371
Muser B, Sommer S, Wolf H, Wehner R (2005) Foraging ecology of the thermophilic Australian desert ant, Melophorus bagoti. Aust J Zool 53:301–311
Narendra A (2007) Homing strategies of the Australian desert ant Melophorus bagoti I: proportional path integration takes the ant half-way home. J Exp Biol 210:1798–1803
Narendra A, Si A, Sulikowski D, Cheng K (2007) Learning, retention and coding of nest-associated visual cues by the Australian desert ant, Melophorus bagoti. Behav Ecol Sociobiol 61:1543–1553
Narendra A, Cheng K, Sulikowski D, Wehner R (2008) Search strategies of ants in landmark-rich habitats. J Comp Physiol A 194:929–938
Narendra A, Gourmaud S, Zeil J (2013) Mapping the navigational knowledge of individually foraging ants, Myrmecia croslandi. Proc R Soc B 280:20130683
Nicholson DJ, Judd PD, Cartwright BA, Collett TS (1999) Learning walks and landmark guidance in wood ants (Formica rufa). J Exp Biol 202:1831–1838
O’Keefe J, Dostrovsky J (1971) The hippocampus as a spatial map: preliminary evidence from unit activity in the freely-moving rat. Brain Res 34:171–175
O’Keefe J, Nadel L (1978) The hippocampus as a cognitive map. Clarendon Press, Oxford
Olton DS, Samuelson RJ (1976) Remembrance of places passed: spatial memory in rats. J Exp Psychol Anim Behav Process 2:97–116
Osborne JL, Smith A, Clark SJ, Reynolds DR, Barron MC, Lim KS, Reynolds AM (2013) The ontogeny of bumblebee flight trajectories: from naive explorers to experienced foragers. PLoS ONE 8:e78681
Pearce JM, Bouton ME (2001) Theories of associative learning in animals. Annu Rev Psychol 52:111–139
Potegal M (1982) Vestibular and neostriatal contributions to spatial orientation. In: Potegal M (ed) Spatial abilities: development and physiological foundations. Academic Press, New York, pp 361–387
Raichlen DA, Wood BM, Gordon AD, Mabulla AZP, Marlowe FW, Pontzer H (2014) Evidence of Lévy walk foraging patterns in human hunter-gatherers. Proc Natl Acad Sci USA 111:728–733
Reynolds AM (2014) Mussels realize Weierstrassian Lévy walks as composite correlated random walks. Sci Rep 4:4409
Reynolds AM, Rhodes CJ (2009) The Lévy flight paradigm: random search patterns and mechanisms. Ecol 90:877–887
Reynolds AM, Smith AD, Menzel R, Greggers U, Reynolds DR, Riley JR (2007a) Displaced honey bees perform optimal scale-free search flights. Ecology 88:1955–1961
Reynolds AM, Smith AD, Reynolds DR, Carreck NL, Osborne JL (2007b) Honeybees perform optimal scale-free searching flights when attempting to locate a food source. J Exp Biol 210:3763–3770
Reynolds AM, Schultheiss P, Cheng K (2013) Are Lévy flight patterns derived from the Weber–Fechner law in distance estimation? Behav Ecol Sociobiol 67:1219–1226
Reynolds AM, Schultheiss P, Cheng K (2014) Does the Australian desert ant Melophorus bagoti approximate a Lévy search by an intrinsic bi-modal walk? J Theor Biol 340:17–22
Rich PD, Liaw H-P, Lee AK (2014) Large environments reveal the statistical structure governing hippocampal representations. Science 345:814–817
Ritchie BF (1947) Studies in spatial learning III: two paths to the same location and two paths to two different locations. J Exp Psychol 37:25–38
Roitblat HL, Bever TG, Terrace HS (1984) Animal cognition. Lawrence Erlbaum Associates, Hillsdale
Ronacher B (2008) Path integration as the basic navigation mechanism of the desert ant Cataglyphis fortis (Forel, 1902) (Hymenoptera: Formicidae). Myrmecol News 11:53–62
Ronacher B, Wehner R (1995) Desert ants Cataglyphis fortis use self-induced optic flow to measure distances travelled. J Comp Physiol A 177:21–27
Ronacher B, Gallizzi K, Wohlgemuth S, Wehner R (2000) Lateral optic flow does not influence distance estimation in the desert ant Cataglyphis fortis. J Exp Biol 203:1113–1121
Rossel S, Wehner R (1986) Polarization vision in bees. Nature 323:128–131
Samsonovich A, McNaughton B (1997) Path integration and cognitive mapping in a continuous attractor neural netwok model. J Neurosci 17:5900–5920
Santschi F (1911) Sur le mécanisme de l’orientation chez les fourmis. Revue Suisse Zool 19:303–338
Schultheiss P, Cheng K (2011) Finding the nest: inbound searching behaviour in the Australian desert ant, Melophorus bagoti. Anim Behav 81:1031–1038
Schultheiss P, Cheng K (2013) Finding food: outbound searching behavior in the Australian desert ant Melophorus bagoti. Behav Ecol 24:128–135
Schultheiss P, Nooten SS (2013) Foraging patterns and strategies in an Australian desert ant. Austral Ecol 38:942–951
Schultheiss P, Schwarz S, Cheng K, Wehner R (2012) Foraging ecology of an Australian salt-pan desert ant (genus Melophorus). Aust J Zool 60:311–319
Schultheiss P, Wystrach A, Legge ELG, Cheng K (2013) Information content of visual scenes influences systematic search of desert ants. J Exp Biol 216:742–749
Schultheiss P, Cheng K, Reynolds AM (2015) Searching behavior in social Hymenoptera. Learn Motiv (in press)
Séguinot V, Maurer R, Etienne AS (1993) Dead reckoning in a small mammal: the evaluation of distance. J Comp Physiol A 173:103–113
Shettleworth SJ (2010) Cognition, evolution, and behavior, 2nd edn. Oxford University Press, New York
Sheynikhovich D, Chavarriaga R, Strösslin T, Arleo A, Gerstner W (2009) Is there a geometric module for spatial orientation? Insights from a rodent navigational model. Psychol Rev 116:540–566
Sims DW, Reynolds AM, Humphries NE, Southall EJ, Wearmouth VJ, Metcalfe B, Twitchett RJ (2014) Hierarchical random walks in trace fossils and the origin of optimal search behavior. Proc Natl Acad Sci USA 111:11073–11078
Spelke ES, Kinzler KD (2007) Core knowledge. Dev Sci 10:89–96
Spetch ML, Cheng K, MacDonald SE, Linkenhoker BA, Kelly DM, Doerkson SR (1997) Use of landmark configuration in pigeons and humans II: generality across search tasks. J Comp Psychol 111:14–24
Srinivasan MV (2011) Honeybees as a model for the study of visually guided flight, navigation, and biologically inspired robotics. Physiol Rev 91:413–460
Srinivasan MV, Zhang SW, Bidwell NJ (1997) Visually mediated odometry in honeybees. J Exp Biol 200:2513–2522
Srinivasan MV, Zhang S, Altwein M, Tautz J (2000) Honeybee navigation: nature and calibration of the “odometer”. Science 287:757–920
Steck K, Hansson BS, Knaden M (2009) Smells like home: desert ants, Cataglyphis fortis, use olfactory landmarks to pinpoint the nest. Front Zool 6:5
Stieb SM, Hellwig A, Wehner R, Rössler W (2010a) Visual experience affects both behavioral and neuronal aspects in the individual life history of the desert ant Cataglyphis fortis. Dev Neurobiol 72:729–742
Stieb SM, Muenz TS, Wehner R, Rössler W (2010b) Visual experience and age affect synaptic organization in the mushroom bodies of the desert ant Cataglyphis fortis. Dev Neurobiol 70:408–423
Stieb SM, Kelber C, Wehner R, Rössler W (2011) Antennal-lobe organization in desert ants of the genus Cataglyphis. Brain Behav Evol 77:136–146
Stone T, Mangan M, Ardin P, Webb B (2014) Sky segmentation with ultraviolet images can be used for navigation. In: Proceedings of the 2014 Robotics: Science and Systems Conference X. http://roboticsproceedings.org/rss10/index.html
Stürzl W, Zeil J (2007) Depth, contrast and view-based homing in outdoor scenes. Biol Cybern 96:519–531
Taube JS (1998) Head direction cells and the neurophysiological basis for a sense of direction. Prog Neurobiol 55:225–256
Taube JS (2007) The head direction signal: origins and sensory-motor integration. Annu Rev Neurosci 30:181–207
Taube JS, Muller RU, Ranck JB Jr (1990a) Head-direction cells recorded from the postsubiculum in freely moving rats I: description and quantitative analysis. J Neurosci 10:420–435
Taube JS, Muller RU, Ranck JB Jr (1990b) Head-direction cells recorded from the postsubiculum in freely moving rats II: effects of environmental manipulations. J Neurosci 10:436–447
Tchernichovski O, Benjamini Y, Golani I (1998) The dynamics of long-term exploration in the rat-part I: a phase-plane analysis of the relationship between location and velocity. Biol Cybern 78:423–432
Teichroeb JA, Chapman CA (2014) Sensory information and associative cues used in food detection by wild vervet monkeys. Anim Cogn 17:517–528
Tinbergen N (1932) Über die Orientierung des Bienenwolfes (Philanthus triangulum, Fabr.). Z Vergl Physiol 16:305–334
Tolman EC (1948) Cognitive maps in rats and men. Psychol Rev 55:189–208
Towne WF (2008) Honeybees can learn the relationship between the solar ephemeris and a newly-experienced landscape. J Exp Biol 211:3737–3743
Towne WF, Moscrip H (2008) The connection between landscapes and the solar ephemeris in honeybees. J Exp Biol 211:3729–3736
Trullier O, Wiener SI, Berthoz A, Meyer JA (1997) Biologically based artificial navigation systems: review and prospects. Prog Neurobiol 51:483–544
Vickerstaff RJ, Cheung A (2010) Which coordinate system for modelling path integration? J Theor Biol 263:242–261
Vickerstaff RJ, Di Paolo EA (2005) Evolving neural models of path integration. J Exp Biol 208:3349–3366
Viswanathan GM, Afanasyev V, Buldyrev SV, Murphy EJ, Prince PA, Stanley HE (1996) Lévy flight search patterns of wandering albatrosses. Nature 381:413–415
Viswanathan GM, Buldyrev SV, Havlin S, da Luz MGE, Raposo EP, Stanley HE (1999) Optimizing the success of random searches. Nature 401:911–914
von Frisch K (1948) Gelöste und ungelöste Rätsel der Bienensprache. Naturwissenschaften 35:38–43
von Frisch K (1949) Die Polarisation des Himmelslichtes als orientierender Faktor bei den Tänzen der Bienen. Experientia 5:142–148
von Frisch K (1953) The dancing bees. Harcourt, Brace & World, New York
von Frisch K (1967) The dance language and orientation of bees. Belknap, Cambridge
von Frisch K, Lindauer M (1954) Himmel und Erde in Konkurrenz bei der Orientierung der Bienen. Naturwissenschaften 41:245–253
Wang RF, Spelke ES (2002) Human spatial representation: insights from animals. Trends Cogn Sci 6:376–382
Warrant E, Dacke M (2011) Vision and visual navigation in nocturnal insects. Annu Rev Entomol 56:239–254
Webb B (2000) What does robotics offer animal behaviour? Anim Behav 60:545–558
Wehner R (1967) Pattern recognition in bees. Nature 215:1244–1248
Wehner R (1968) Optische Orientierungsmechanismen im Heimkehr-Verhalten von Cataglyphis bicolor (Formicidae, Hymenoptera). Revue Suisse Zool 75:1076–1085
Wehner R (1987a) ‘Matched filters’: neural models of the external world. J Comp Physiol A 161:511–531
Wehner R (1987b) Spatial organization of the foraging behavior in individually searching desert ants, Cataglyphis (Sahara desert) and Ocymyrmex (Namib desert). In: Pasteels JM, Deneubourg JM (eds) From individual to collective behavior in insects. Birkhäuser, Basel, pp 15–42
Wehner R (1994) The polarization-vision project: championing organismic biology. Fortschritte Zool 39:103–143
Wehner R (1997) The ant’s celestial compass system: spectral and polarization channels. In: Lehrer M (ed) Orientation and communication in arthropods. Birkhäuser, Basel, pp 145–185
Wehner R (2003) Desert ant navigation: how miniature brains solve complex tasks. J Comp Physiol A 189:579–588
Wehner R (2009) The architecture of the desert ant’s navigational toolkit (Hymenoptera: Formicidae). Myrmecol News 12:85–96
Wehner R (2013) Life as a cataglyphologist: and beyond. Annu Rev Entomol 58:1–18
Wehner R, Müller M (2006) The significance of direct sunlight and polarized skylight in the ant’s celestial system of navigation. Proc Natl Acad Sci USA 103:12575–12579
Wehner R, Räber F (1979) Visual spatial memory in desert ants, genus Cataglyphis (Formicidae, Hymenoptera). Experientia 35:1569–1571
Wehner R, Srinivasan MV (1981) Searching behaviour of desert ants, genus Cataglyphis (Formicidae, Hymenoptera). J Comp Physiol A 142:315–338
Wehner R, Srinivasan MV (2003) Path integration in insects. In: Jeffery KJ (ed) The neurobiology of spatial behaviour. Oxford University Press, Oxford, pp 9–30
Wehner R, Wehner S (1990) Insect navigation: use of maps or Ariadne’s thread. Ethol Ecol Evol 2:27–48
Wehner R, Harkness RD, Schmid-Hempel P (1983) Foraging strategies in individually searching ants Cataglyphis bicolor (Hymenoptera: Formicidae). Gustav Fischer Verlag, Stuttgart
Wehner R, Marsh AC, Wehner S (1992) Desert ants on a thermal tightrope. Nature 357:586–587
Wehner R, Michel B, Antonsen P (1996) Visual navigation in insects: coupling of egocentric and geocentric information. J Exp Biol 199:129–140
Wehner R, Meier C, Zollikofer C (2004) The ontogeny of foraging behaviour in desert ants, Cataglyphis bicolor. Ecol Entomol 29:240–250
Wehner R, Boyer M, Loertscher F, Sommer S, Menzi U (2006) Ant navigation: one-way routes rather than maps. Curr Biol 16:75–79
West SA, El Mouden C, Gardner A (2011) Sixteen common misconceptions about the evolution of cooperation in humans. Evol Hum Behav 32:231–262
Wiener J, Shettleworth S, Bingman VP, Cheng K, Healy S, Jacobs LF, Jeffery KJ, Mallot HA, Menzel R, Newcombe NS (2011) Animal navigation: a synthesis. In: Menzel R, Fischer J (eds) Animal thinking: contemporary issues in comparative cognition. MIT Press, Cambridge, pp 51–76
Wittlinger M, Wehner R, Wolf H (2006) The ant odometer: stepping on stilts and stumps. Science 312:1965–1967
Wittlinger M, Wehner R, Wolf H (2007) The desert ant odometer: a stride integrator that accounts for stride length and walking speed. J Exp Biol 210:198–207
Wittmann T, Schwegler H (1995) Path integration: a network model. Biol Cybern 73:569–575
Wolf H (2011) Odometry and insect navigation. J Exp Biol 214:1629–1641
Wystrach A, Beugnon G, Cheng K (2011) Landmarks or panoramas: what do navigating ants attend to for guidance? Front Zool 8:21
Wystrach A, Beugnon G, Cheng K (2012) Ants might use different view-matching strategies on and off the route. J Exp Biol 215:44–55
Wystrach A, Mangan M, Philippides A, Graham P (2013a) Snapshots in ants? New interpretations of paradigmatic experiments. J Exp Biol 216:1766–1770
Wystrach A, Schwarz S, Baniel A, Cheng K (2013b) Backtracking behaviour in lost ants: an additional strategy in their navigational toolkit. Proc R Soc B 280:20131677
Wystrach A, Philippides A, Aurejac A, Cheng K, Graham P (2014a) Visual scanning behaviours and their role in the navigation of the Australian desert ant Melophorus bagoti. J Comp Physiol A 200:615–626
Wystrach A, Schwarz S, Schultheiss P, Baniel A, Cheng K (2014b) Multiple sources of celestial compass information in the Central Australian desert ant Melophorus bagoti. J Comp Physiol A 200:591–601
Zeil J (1993a) Orientation flights of solitary wasps (Cerceris; Sphecidae; Hymenoptera) I: description of flight. J Comp Physiol A 172:189–205
Zeil J (1993b) Orientation flights of solitary wasps (Cerceris; Sphecidae; Hymenoptera) II: similarities between orientation and return flights and the use of motion parallax. J Comp Physiol A 172:207–222
Zeil J (2012) Visual homing: an insect perspective. Curr Opin Neurobiol 22:285–293
Zeil J, Hemmi JM (2006) The visual ecology of fiddler crabs. J Comp Physiol A 192:1–25
Zeil J, Zanker JM (1997) A glimpse into crabworld. Vision Res 37:3417–3426
Zeil J, Hofmann MI, Chahl JS (2003) Catchment areas of panoramic snapshots in outdoor scenes. J Opt Soc Am 20:450–469
Zeil J, Narendra A, Stürzl W (2014) Looking and homing: how displaced ants decide where to go. Phil Trans R Soc B 369:20130034
Acknowledgments
We wish to thank Tom Collett, Sibylle Wehner, and an anonymous reviewer for comments on this manuscript.
Conflict of interst
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cheng, K., Freas, C.A. Path integration, views, search, and matched filters: the contributions of Rüdiger Wehner to the study of orientation and navigation. J Comp Physiol A 201, 517–532 (2015). https://doi.org/10.1007/s00359-015-0984-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-015-0984-9