Abstract
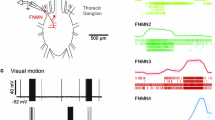
Recent behavioural studies have demonstrated that honeybees use visual feedback to stabilize their gaze. However, little is known about the neural circuits that perform the visual motor computations that underlie this ability. We investigated the motor neurons that innervate two neck muscles (m44 and m51), which produce stabilizing yaw movements of the head. Intracellular recordings were made from five (out of eight) identified neuron types in the first cervical nerve (IK1) of honeybees. Two motor neurons that innervate muscle 51 were found to be direction-selective, with a preference for horizontal image motion from the contralateral to the ipsilateral side of the head. Three neurons that innervate muscle 44 were tuned to detect motion in the opposite direction (from ipsilateral to contralateral). These cells were binocularly sensitive and responded optimally to frontal stimulation. By combining the directional tuning of the motor neurons in an opponent manner, the neck motor system would be able to mediate reflexive optomotor head turns in the direction of image motion, thus stabilising the retinal image. When the dorsal ocelli were covered, the spontaneous activity of neck motor neurons increased and visual responses were modified, suggesting an ocellar input in addition to that from the compound eyes.










Similar content being viewed by others
References
Baader A (1991) The contribution of some neck and abdominal motoneurones in locust (Locusta migratoria) steering reactions. J Insect Physiol 37:689–697
Baird E, Srinivasan MV, Zhang S, Cowling A (2005) Visual control of flight speed in honeybees. J Exp Biol 208:3895–3905
Batschelet E (1981) Circular statistics in biology. Academic Press, London
Berry R, Ibbotson M (2010) A three-dimensional atlas of the honeybee neck. PLoS ONE 5(5):1–14
Berry R, Stange G, Olberg R, van Kleef J (2006) The mapping of visual space by identified large second-order neurons in the dragonfly median ocellus. J Comp Physiol A 192:1105–1123
Böddeker N, Hemmi JM (2010) Visual gaze control during peering flight manoeuvres in honeybees. Proc R Soc B Biol Sci 277:1209–1217
Böddeker N, Dittmar L, Stuerzl W, Egelhaaf M (2010) The fine structure of honeybee head and body yaw movements in a homing task. Proc R Soc B Biol Sci 277:1899–1906
Cajal SR (1918) Observaciones sobre la estructura de los ocelos y vias nerviosas ocelars de algunos insectos. Trab Lab Invest Biol Ubiv Madrid 16:109–139
Chiappe M, Eugenia SJD, Reiser MB, Jayaraman V (2010) Walking modulates speed sensitivity in Drosophila motion vision. Curr Biol 20:1470–1475
Fuchs AF, Luschei ES (1970) Firing patterns of abducens neurons of alert monkeys in relationship to horizontal eye movement. J Neurophysiol 33:382–390
Gilbert C, Gronenberg W, Strausfeld NJ (1995) Oculomotor control in calliphorid flies - head movements during activation and inhibition of neck motor-neurons corroborate neuroanatomical predictions. J Comp Neurol 361:285–297
Goodman LJ (1981) Organisation and physiology of the insect dorsal ocellar system. In: Autrum H (ed) Handbook of sensory physiology, vol VII/6C. Springer, Berlin, pp 201–286
Hausen K (1982) Motion sensitive interneurons in the optomotor system of the fly.2. The horizontal cells—receptive-field organization and response characteristics. Biological Cybernetics 46:67–79
Hengstenberg R (1984) Roll-stabilization during flight of the blowfly’s head and body by mechanical and visual cues. In: Varju D, Schnitzler U (eds) Localization and orientation in biology and engineering. Springer, Berlin, pp 121–134
Hengstenberg R, Sandeman DC, Hengstenberg B (1986) Compensatory head roll in the blow fly Calliphora during flight. Proc R Soc Lond B 227:455–482
Huston SJ, Krapp HG (2008) Visuomotor transformation in the fly gaze stabilization system. PLOS Biol 6:1468–1478
Huston SJ, Krapp HG (2009) Nonlinear integration of visual and haltere inputs in fly neck motor neurons. J Neurosci 29:13097–13105
Ibbotson MR (1991) A motion-sensitive visual descending neurone in Apis mellifera monitoring translatory flow-fields in the horizontal plane. J Exp Biol 157:573–577
Ibbotson MR, Crowder NA, Cloherty SL, Price NSC, Mustari MJ (2008) Saccadic modulation of neural responses: possible roles in saccadic suppression, enhancement and time compression. J Neurosci 28:10952–10960
Ibbotson MR, Goodman LJ (1990) Response characteristics of four wide-field motion-sensitive descending interneurons in Apis mellifera. J Exp Biol 148:255–279
Ibbotson MR, Mark RF (1996) Impulse responses distinguish two classes of directional motion-sensitive neurons in the nucleus of the optic tract. J Neurophysiol 75:996–1007
Ibbotson MR, Price NSC (2001) Spatiotemporal tuning of directional neurons in mammalian and avian pretectum: a comparison of physiological properties. J Neurophysiol 86:2621–2624
Ibbotson MR, Price NSC, Crowder NA, Ono S, Mustari MJ (2007) Enhanced motion sensitivity follows saccadic suppression in the superior temporal sulcus of the macaque cortex. Cereb Cortex 17:1129–1138
Karmeier K, van Hateren JH, Kern R, Egelhaaf M (2006) Encoding of naturalistic optic flow by a population of blowfly motion-sensitive neurons. J Neurophysiol 96:1602–1614
Kern R, van Hateren JH, Michaelis C, Lindemann JP, Egelhaaf M (2005) Function of a fly motion-sensitive neuron matches eye movements during free flight. Plos Biol 3:1130–1138
Krapp HG, Hengstenberg R (1996) Estimation of self-motion by optic flow processing in single visual interneurons. Nature 384:463–466
Kunze P (1961) Untersuchung des bewegungssehens fixiert fliegender bienen. Zeitschrift fur Vergleichende Physiologie 44:656–684
Land MF (1973) Head movements of flies during visually guided flight. Nature 243:299–300
Lindemann JP, Kern R, Michaelis C, Meyer P, van Hateren JH, Egelhaaf M (2003) Flimax, a novel stimulus device for panoramic and highspeed presentation of behaviourally generated optic flow. Vis Res 43:779–791
Lindemann JP, Weiss H, Moeller R, Egelhaaf M (2008) Saccadoc flight strategy facilitates collision avoidance: closed-loop performance of a cyberfly. Biol Cybern 98:213–227
Longden K, Krapp H (2009) State-dependent performance of optic flow processing interneurons. J Neurophysiol 102:3606–3618
Maimon G, Straw AD, Dickinson MH (2010) Active flight increases the gain of visual motion processing in Drosophila. Nat Neurosci 13:U29–U393
Markl H (1966) Peripheres Nervensystem und Muskulatur im Thorax der Arbeiterin von Apis mellifica L., Formica polyctena Foerster und Vespa vulgaris L. und der Grundplan der Innervierung des Insektenthorax. Zool Jb Anat 83:107–184
Menzel R, Hammer M, Braun G, Mauelshagen J, Sugawa M (1991) Neurobiology of learning and memory in honeybees. In: Goodman L, Fisher R (eds) The behaviour and physiology of bees. CAB International, Wallingford, pp 323–353
Milde J (1981) Graded potentials and action potentials in the large ocellar interneurons of the bee. J Comp Physiol A 143:427–434
Milde J, Seyan H, Strausfeld NJ (1987) The neck motor system of the fly Calliphora erythrocephala II. Sensory organization. J Comp Physiol A 160:225–238
O’Carroll DC, Bidwell NJ, Laughlin SB, Warrant E (1996) Insect motion detectors matched to visual ecology. Nature 382:63–66
Parsons MM, Krapp HG, Laughlin SB (2010) Sensor fusion in identified visual interneurons. Curr Biol 20:624–628
Reichert H, Rowell CHF, Griss C (1985) Course correction circuitry translates feature detection into behavioural action in locusts. Nature 315:142–144
Reichert H, Rowell CHF (1986) Neuronal circuits controlling flight in the locust: how sensory information is processed for motor control. TINs 9:281–283
Ribi WA (1975a) The first optic ganglion of the bee. I. Correlation between visual cell types and their terminals in the lamina and medulla. Cell Tissue Res 165:103–111
Ribi WA (1975b) The neurons in the first optic ganglion of the bee (Apis mellifera). Adv Anat Embryol Cell Biol 50:1–43
Schröter U, Wilson S, Srinivasan MV, Ibbotson MR (2007) The morphology and physiology of suboesophageal neck motoneurons in the honeybee. J Comp Physiol A 193:289–304
Snodgrass RE (1942) The skeleto-muscular mechanisms of the honey bee. Smithson Misc Collect 103:1–120
Sobel EC (1990) The locust’s use of motion parallax to measure distance. J Comp Physiol 167:579–588
Srinivasan M, Zhang S, Lehrer M, Collett T (1996) Honeybee navigation en route to the goal: visual flight control and odometry. J Exp Biol 199:237–244
Strausfeld NJ, Seyan H, Milde JJ (1987) The neck motor system of the fly Calliphora erythrocephala I. Muscles and motor neurons. J Comp Physiol A 160:205–224
van Hateren JH, Schilstra C (1999) Blowfly flight and optic flow II. Head movements during flight. J Exp Biol 202:1491–1500
Wertz A, Gaub B, Plett J, Haag J, Borst A (2009a) Robust coding of ego-motion in descending neurons of the fly. J Neurosci 29:14993–15000
Wertz A, Haag J, Borst A (2009b) Local and global motion preferences in descending neurons of the fly. J Comp Physiol 195:1107–1120
Acknowledgments
To our knowledge, the first people to record from neurons in IK1 were Christopher J. Pomfrett and Lesley J. Goodman (unpublished observations). This preliminary work motivated the present study. The LED-based visual stimulator was developed by Dr Gert Stange. Dr Richard Berry developed the three-dimensional model of the bee neck used to generate Fig. 1a, b.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hung, YS., van Kleef, J.P. & Ibbotson, M.R. Visual response properties of neck motor neurons in the honeybee. J Comp Physiol A 197, 1173–1187 (2011). https://doi.org/10.1007/s00359-011-0679-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-011-0679-9