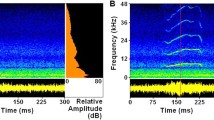
Abstract
Territorial males of the pan-Amazonian Dart-poison frog, Epipedobates femoralis, are known to present stereotypic phonotactic responses to the playback of conspecific and synthetic calls. Fixed site attachment and a long calling period within an environment of little temperature change render this terrestrial and diurnal pan-Amazonian frog a rewarding species for field bioacoustics. In experiments at the field station Arataï, French Guiana, we tested whether the prominent frequency modulation of the advertisement-call notes is critical for eliciting phonotactic responses. Substitution of the natural upward sweep by either a pure tone within the species frequency range or a reverse sweep did not alter the males’ phonotactic behavior. Playbacks with artificial advertisement calls embedded in high levels of either low-pass or high-pass masking noise designed to saturate nerve fibers from either the amphibian papilla or basilar papilla showed that male phonotactic behavior in this species is subserved by activation of the basilar papilla of the inner ear.





Similar content being viewed by others
Abbreviations
- FM :
-
Frequency modulated
- AP :
-
Amphibian papilla
- BP :
-
Basilar papilla
References
Doherty JA, Gerhardt HC (1984) Evolutionary and neurobiological implications of selective phonotaxis in the spring peeper (Hyla crucifer). Anim Behav 51:981–989
Ellinger N, Hödl W (2003) Habitat acoustics of a neotropical lowland forest. Bioacoustics 13:297–321
Feer F (1999) Effects of dung beetles (Scarabaeidae) on seeds dispersed by howler monkeys (Alouatta seniculus) in the French Guianan rain forest. J Trop Ecol 15:129–142
Feng AS, Hall JC, Gooler DM (1990) Neural basis of sound pattern recognition in anurans. Prog Neurobiol 34:313–329
Feng AS, Narins PM, Xu C-H (2002) Vocal acrobatics in a Chinese frog, Amolops tormotus. Naturwissenschaften 89:352–356.
Gerhardt HC (1978) Temperature coupling in the vocal communication system of the gray treefrog Hyla versicolor. Science 199:992–994
Gerhardt HC (1988) Acoustic properties used in call recognition by frogs and toads. In: Fritzsch B, Ryan MJ, Wilczynski W, Hetherington TE, Walkowiak W (eds) The evolution of the amphibian auditory system. Wiley, New York, pp 455–483
Gerhardt HC, Doherty JA (1988) Acoustic communication in the gray treefrog, Hyla versicolor: evolutionary and neurobiological implications. J Comp Physiol A 162:261–278
Gerhardt HC, Huber F (2002) Acoustic communication in insects and anurans. University of Chicago Press, Chicago
Gerhardt HC, Rheinlaender J (1980) Accuracy of sound localization in a miniature dendrobatid frog. Naturwissenschaften 67:362–363
Gottsberger B, Gruber E (2004) Temporal partitioning of reproductive activity in a Neotropical anuran community. J Trop Ecol 20:271–280
Grafe TU (1996) The function of call alternation in the African reed frog (Hyperolius marmarotus): precise call timing prevents auditory masking. Behav Ecol Sociobiol 38:149–158
Hödl W (1982) Phyllobates femoralis (Dendrobatidae): Rufverhalten und akustische Orientierung der Männchen (Freilandaufnahmen). Scientific film Ctf 1788 BHWK Wien
Hödl W (1983) Phyllobates femoralis (Dendrobatidae): Rufverhalten und akustische Orientierung der Männchen (Freilandaufnahmen). Acompanying paper to scientific film Ctf 1788 BHWK Wien; Wiss Film 30:12–19
Hödl W (1987) Dendrobates femoralis (Dendrobatidae): a handy fellow for frog bioacoustics. In: Proceedings of 4th Ord Gen Mtg Soc Eur Herpetol, pp 201–204
Lewis ER, Narins PM (1999) The acoustic periphery of amphibians: anatomy and physiology. In: Fay RR, Popper AN (eds) Comparative hearing: fish and amphibians. Springer, Berlin Heidelberg New York, pp 101–154
Lewis ER, Narins PM, Cortopassi KA, Yamada WM, Poinar EH, Moore SW, Yu XL (2001) Do male white-lipped frogs use seismic signals for intraspecific communication? Am Zool 41:1185–1199
Lüddecke H (1974) Ethologische Untersuchungen zur Fortpflanzung von Phyllobates palmatus (Amphibia, Ranidae). PhD Thesis, Johannes Gutenberg University
Narins PM (1982) Effects of masking noise on evoked calling in the Puerto Rican coqui (Anura: Leptodactylidae). J Comp Physiol 147:439–446
Narins PM (1983) Synchronous vocal response mediated by the amphibian papilla in a neotropical treefrog: behavioral evidence. J Exp Biol 105:95–105
Narins PM, Capranica RR (1980) Neural adaptations for processing the two-note call of the Puerto Rican treefrog, Eleutherodactylus coqui. Brain Behav Evol 17:48–66
Narins PM, Feng AS, Yong H-S, Christensen-Dalsgaard J (1998) Morphological, behavioral and genetic divergence of sympatric morphotypes of the treefrog Polypedates leucomystax in Peninsular Malaysia. Herpetologica 54:129–142
Narins PM, Hödl W, Grabul DS (2003) Bimodal signal requisite for agonistic behavior in a Dart-poison frog, Epipedobates femoralis. Proc Natl Acad Sci USA 100:577–580
Narins PM, Lewis ER, McClelland BE (2000) Hyperextended call repertoire of the endemic Madagascar treefrog, Boophis madagascariensis (Rhacophoridae). J Zool 250:283–298
Narins PM, Zelick R (1988) The effects of noise on auditory processing and behavior in amphibians. In: Fritzsch B, Ryan MJ, Wilczynski W, Hetherington TE, Walkowiak W (eds) The evolution of the amphibian auditory system. Wiley, New York, pp 511–536
Roithmair M (1992) Territoriality and male mating success in the Dart-poison frog, Epipedobates femoralis (Dendrobatidae, Anura). Ethology 92:331–343
Rand S (1988) An overview of anuran acoustic communication. In: Fritzsch B, Ryan MJ, Wilczynski W, Hetherington TE, Walkowiak W (eds) The evolution of the amphibian auditory system. Wiley, New York, pp 415–431
Ryan MJ (1985) The túngara frog. Chicago University Press, Chicago
Ryan MJ (1986) Synchronized calling in a treefrog (Smilisca sila). Brain Behav Evol 29:196–206
Schlüter A (1980) Bioakustische Untersuchungen an Dendrobatiden in einem begrenzten Gebiet des tropischen Regenwaldes von Peru (Amphibia: Salientia: Dendrobatidae). Salamandra 16:149–161
Schwartz JJ (1986) Male calling behavior and female choice in the neotropical treefrog Hyla microcephala. Ethology 73:116–127
Schwartz JJ (1987) The importance of spectral and temporal properties in species and call recognition in a neotropical treefrog with a complex vocal repertoire. Anim Behav 35:340–347
Schwartz JJ, Wells KD (1984) Interspecific acoustic interactions of the neotropical treefrog Hyla ebraccata. J Comp Physiol 14:21–224
Suga N (1972) Analysis of information-bearing elements in complex sounds by auditory neurons of bats. Audiology 11:58–72
Vences M, Kosuch J, Lötters S, Widmer A, Jungfer KH, Köhler J, Veith M (2000) Phylogeny and classification of poison frogs (Amphibia: Dendrobatidae), based on mitochondrial 16S and 12S ribosomal RNA gene sequences. Mol Phylogenet Evol 15:34–40
Wells KD (1977) The social behavior of anuran amphibians. Anim Behav 25:666–693
Wells KD (1988) The effect of social interactions on anuran vocal behavior. In: Fritzsch B, Ryan MJ, Wilczynski W, Hetherington TE, Walkowiak W (eds) The evolution of the amphibian auditory system. Wiley, New York, pp 433–454
Wilczynski W, Rand AS, Ryan MJ (1995) The processing of spectral cues by the call analysis system of the túngara frog, Physalaemus pustulosus. Anim Behav 49:911–929
Zakon HH, Wilczynski W (1988) The physiology of the anuran eighth nerve. In: Fritzsch B, Ryan MJ, Wilczynski W, Hetherington TE, Walkowiak W (eds) The evolution of the amphibian auditory system. Wiley, New York, pp 125–155
Zelick RD, Narins PM (1982) Analysis of acoustically-evoked call suppression behavior in a neotropical treefrog. Anim Behav 30:728–733
Zimmermann H, Zimmermann E (1988) Ethotaxonomie und zoogeographische Artengruppenbildung bei Pfeilgiftfröschen (Anura: Dendrobatidae). Salamandra 24:125–160
Acknowledgements
We thank Vanessa Hequet, Muriel Nugent, the Association Arataï and especially Philippe Gaucher for their unfailing and generous assistance with this project. Brigitte Gottsberger, Daniela Grabul, Edith Gruber, and Christian Proy assisted during the field experiments. Norbert Ellinger, Herbert Gasser and Daniela Grabul synthesized the playback signals. The study was supported by grants from the Austrian Science Foundation (FWF Projects P 11565, P 15345) to W.H. and by NIH Grant no. DC00222 and Academic Senate grant 3501 to P.M.N.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hödl, W., Amézquita, A. & Narins, P.M. The rôle of call frequency and the auditory papillae in phonotactic behavior in male Dart-poison frogs Epipedobates femoralis (Dendrobatidae). J Comp Physiol A 190, 823–829 (2004). https://doi.org/10.1007/s00359-004-0536-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-004-0536-1