Abstract
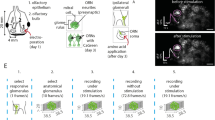
The insect antennal lobe is the first brain structure to process olfactory information. Like the vertebrate olfactory bulb the antennal lobe is substructured in olfactory glomeruli. In insects, glomeruli can be morphologically identified, and have characteristic olfactory response profiles. Local neurons interconnect glomeruli, and output (projection) neurons project to higher-order brain centres. The relationship between their elaborate morphology and their physiology is not understood. We recorded electrophysiologically from antennal lobe neurons, and iontophoretically injected a calcium-sensitive dye. We then measured their spatio-temporal calcium responses to a variety of odours. Finally, we confocally reconstructed the neurons, and identified the innervated glomeruli. An increase or decrease in spiking frequency corresponded to an intracellular calcium increase or decrease in the cell. While intracellular recordings generally lasted between 10 and 30 min, calcium imaging was stable for up to 2 h, allowing a more detailed physiological analysis. The responses indicate that heterogeneous local neurons get input in the glomerulus in which they branch most strongly. In many cases, the physiological response properties of the cells corresponded to the known response profile of the innervated glomerulus. In other words, the large variety of response profiles generally found when comparing antennal lobe neurons is reduced to a more predictable response profile when the innervated glomerulus is known.








Similar content being viewed by others
Abbreviations
- ACT:
-
antenno-cerebralis-tract
- AL:
-
antennal lobe
- AP:
-
action potential
- l-ACT:
-
lateral ACT
- LN:
-
local neuron
- LPL:
-
lateral protocerebral lobe
- m-ACT:
-
medial ACT
- MB:
-
mushroom body
- OSN:
-
olfactory sensory neuron
- PN:
-
projection neuron
- T1:
-
tract 1 of the antennal nerve
References
Abel R, Rybak J, Menzel R (2001) Structure and response patterns of olfactory interneurons in the honeybee, Apis mellifera. J Comp Neurol 437:363–383
Berg BG, Almaas TJ, Bjaalie JG, Mustaparta H (1998) The macroglomerular complex of the antennal lobe in the tobacco budworm moth Heliothis virescens: specified subdivision in four compartments according to information about biologically significant compounds. J Comp Physiol A 183:669–682
Berg BG, Galizia CG, Brandt R, Mustaparta H (2002) Digital atlases of the antennal lobe in two species of tobacco budworm moths, the oriental Helicoverpa assulta (male) and the American Heliothis virescens (male and female). J Comp Neurol 446:123–134
Bicker G (1999) Histochemistry of classical neurotransmitters in antennal lobes and mushroom bodies of the honeybee. Microsc Res Tech 45:174–183
Boeckh J, Tolbert LP (1993) Synaptic organization and development of the antennal lobe in insects. Microsc Res Tech 24:260–280
Bornhauser BC, Meyer EP (1997) Histamine-like immunoreactivity in the visual system and brain of an orthopteran and a hymenopteran insect. Cell Tissue Res 287:211–221
Bruyne M de, Clyne PJ, Carlson JR (1999) Odor coding in a model olfactory organ: the Drosophila maxillary palp. J Neurosci 19:4520–4532
Bruyne M de, Foster K, Carlson JR( 2001) Odor coding in the Drosophila antenna. Neuron 30:537–552
Charpak S, Mertz J, Beaurepaire E, Moreaux L, Delaney K (2001) Odor-evoked calcium signals in dendrites of rat mitral cells. Proc Natl Acad Sci USA 98:1230–1234
Christensen TA, Waldrop BR, Harrow ID, Hildebrand JG (1993) Local interneurons and information processing in the olfactory glomeruli of the moth Manduca sexta. J Comp Physiol A 173:385–399
Christensen TA, Pawlowski VM, Lei H, Hildebrand JG (2000) Multi-unit recordings reveal context-dependent modulation of synchrony in odor-specific neural ensembles. Nat Neurosci 3:927–931
Distler PG, Boeckh J (1998) An improved model of the synaptic organization of insect olfactory glomeruli. Ann N Y Acad Sci 855:508–510
Faber T, Joerges J, Menzel R (1999) Associative learning modifies neural representations of odors in the insect brain. Nat Neurosci 2:74–78
Flanagan D, Mercer AR (1989a) An atlas and 3-D reconstruction of the antennal lobes in the worker honey bee, Apis mellifera L. (Hymenoptera: Apidae). Int J Insect Morphol Embryol 18:145–159
Flanagan D, Mercer AR (1989b) Morphology and response characteristics of neurones in the deutocerebrum of the brain in the honeybee Apis mellifera. J Comp Physiol A 164:483–494
Fonta C, Sun XJ, Masson C (1993) Morphology and spatial distribution of bee antennal lobe interneurones responsive to odours. Chem Senses 18:101–119
Friedrich RW, Laurent G (2001) Dynamic optimization of odor representations by slow temporal patterning of mitral cell activity. Science 291:889–894
Friedrich RW, Stopfer M (2001) Recent dynamics in olfactory population coding. Curr Opin Neurobiol 11:468–474
Galizia CG, Menzel R (2001) The role of glomeruli in the neural representation of odours: results from optical recording studies. J Insect Physiol 47:115–130
Galizia CG, Joerges J, Kuttner A, Faber T, Menzel R (1997) A semi-in vivo preparation for optical recording of the insect brain. J Neurosci Methods 76:61–69
Galizia CG, Nagler K, Holldobler B, Menzel R (1998) Odour coding is bilaterally symmetrical in the antennal lobes of honeybees (Apis mellifera). Eur J Neurosci 10:2964–2974
Galizia CG, McIlwrath SL, Menzel R (1999a) A digital three-dimensional atlas of the honeybee antennal lobe based on optical sections acquired by confocal microscopy. Cell Tissue Res 295:383–394
Galizia CG, Sachse S, Rappert A, Menzel R (1999b) The glomerular code for odor representation is species specific in the honeybee Apis mellifera. Nat Neurosci 2:473–478
Gascuel J, Masson C (1991) A quantitative ultrastructural study of the honeybee antennal lobe. Tissue Cell 23:341–355
Goldberg F, Grunewald B, Rosenboom H, Menzel R (1999) Nicotinic acetylcholine currents of cultured Kenyon cells from the mushroom bodies of the honey bee Apis mellifera. J Physiol (Lond) 514:759–768
Hansson BS, Anton S (2000) Function and morphology of the antennal lobe: new developments. Annu Rev Entomol 45:203–231
Hansson BS, Christensen TA (1999) Functional characteristics of the antennal lobe. In: Hansson BS (ed) Insect olfaction. Springer, Berlin Heidelberg New York, pp 125–161
Hansson BS, Ljungberg H, Hallberg E, Lofstedt C (1992) Functional specialization of olfactory glomeruli in a moth. Science 256:1313–1315
Hildebrand JG, Shepherd GM (1997) Mechanisms of olfactory discrimination: converging evidence for common principles across phyla. Annu Rev Neurosci 20:595–631
Homberg U (1984) Processing of antennal information in extrinsic mushroom body neurons of the bee brain. J Comp Physiol A 154:825–836
Kanzaki R, Arbas EA, Strausfeld NJ, Hildebrand JG (1989) Physiology and morphology of projection neurons in the antennal lobe of the male moth Manduca sexta. J Comp Physiol A 165:427–453
King JR, Christensen TA, Hildebrand JG (2000) Response characteristics of an identified, sexually dimorphic olfactory glomerulus. J Neurosci 20:2391–2399
Kreissl S, Bicker G (1989) Histochemistry of acetylcholinesterase and immunocytochemistry of an acetylcholine receptor-like antigen in the brain of the honeybee. J Comp Neurol 286:71–84
Kurtz R, Warzecha AK, Egelhaaf M (2001) Transfer of visual motion information via graded synapses operates linearly in the natural activity range. J Neurosci 21:6957–6966
Laissue PP, Reiter C, Hiesinger PR, Halter S, Fischbach KF, Stocker RF (1999) Three-dimensional reconstruction of the antennal lobe in Drosophila melanogaster. J Comp Neurol 405:543–552
Laurent G (1999) A systems perspective on early olfactory coding. Science 286:723–728
Laurent G, Wehr M, Davidowitz H (1996) Temporal representations of odors in an olfactory network. J Neurosci 16:3837–3847
Laurent G, Stopfer M, Friedrich RW, Rabinovich MI, Volkovskii A, Abarbanel HD (2001) Odor encoding as an active, dynamical process: experiments, computation, and theory. Annu Rev Neurosci 24:263–297
MacLeod K, Laurent G (1996) Distinct mechanisms for synchronization and temporal patterning of odor-encoding neural assemblies. Science 274:976–979
Malun D (1991) Inventory and distribution of synapses of identified uniglomerular projection neurons in the antennal lobe of Periplaneta americana. J Comp Neurol 305:348–360
Mazziotta J, Toga A, Evans A, Fox P, Lancaster J, Zilles K, Woods R, Paus T, Simpson G, Pike B, Holmes C, Collins L, Thompson P, MacDonald D, Iacoboni M, Schormann T, Amunts K, Palomero-Gallagher N, Geyer S, Parsons L, Narr K, Kabani N, Le Goualher G, Boomsma D, Cannon T, Kawashima R, Mazoyer B (2001) A probabilistic atlas and reference system for the human brain: international consortium for brain mapping (ICBM). Philos Trans R Soc Lond B 356:1293–1322
Menzel R (2001) Searching for the memory trace in a mini-brain, the honeybee. Learn Mem 8:53–62
Menzel R, Giurfa M (2001) Cognitive architecture of a mini-brain: the honeybee. Trends Cogn Sci 5:62–71
Menzel R, Brandt R, Rohlfing T, Zoeckler M, Stollhoff N (2002) The virtual bee brain atlas—a novel way to document neuroanatomical data. In: XIV International Congress of IUSSI. IUSSI, Sapporo, Japan, p 28
Müller D, Abel R, Brandt R, Zockler M, Menzel R (2002) Differential parallel processing of olfactory information in the honeybee, Apis mellifera L. J Comp Physiol A 188:359–370
Müller U (2000) Prolonged activation of cAMP-dependent protein kinase during conditioning induces long-term memory in honeybees. Neuron 27:159–168
Ng M, Roorda RD, Lima SQ, Zemelman BV, Morcillo P, Miesenbock G (2002) Transmission of olfactory information between three populations of neurons in the antennal lobe of the fly. Neuron 36:463–474
Rospars JP (1988) Structure and development of the insect antennodeutocerebral system. Int J Insect Morphol Embryol 17:243–294
Rospars JP, Chambille I (1981) Deutocerebrum of the cockroach Blaberus craniifer Burm. Quantitative study and automated identification of the glomeruli. J Neurobiol 12:221–247
Røstelien T, Borg-Karlson AK, Fäldt J, Jacobsen U, Mustaparta H (2000) The plant sesquiterpene germacrene D specifically activates a major type of antennal receptor neruons of the tobacco budworm moth Heliothis virescens. Chem Senses 25:141–148
Sachse S, Galizia CG (2002) Role of inhibition for temporal and spatial odor representation in olfactory output neurons: a calcium imaging study. J Neurophysiol 87:1106–1117
Sachse S, Rappert A, Galizia CG (1999) The spatial representation of chemical structures in the antennal lobe of honeybees: steps towards the olfactory code. Eur J Neurosci 11:3970–3982
Sadek MM, Hansson BS, Rospars JP, Anton S (2002) Glomerular representation of plant volatiles and sex pheromone components in the antennal lobe of the female Spodoptera littoralis. J Exp Biol 205:1363–1376
Schäfer S, Bicker G (1986) Distribution of GABA-like immunoreactivity in the brain of the honeybee. J Comp Neurol 246:287–300
Single S, Borst A (2002) Different mechanisms of calcium entry within different dendritic compartments. J Neurophysiol 87:1616–1624
Stocker RF, Lienhard MC, Borst A, Fischbach KF (1990) Neuronal architecture of the antennal lobe in Drosophila melanogaster. Cell Tissue Res 262:9–34
Stopfer M, Bhagavan S, Smith BH, Laurent G (1997) Impaired odour discrimination on desynchronization of odour-encoding neural assemblies. Nature 390:70–74
Stranden M, Borg-Karlson A-K, Mustaparta H (2002) Receptor neuron discrimination of germacrene D enantiomers. Chem Senses 27:143–152
Sun X-J, Fonta C, Masson C (1993) Odour quality processing by bee antennal lobe interneurones. Chem Senses 18:355–377
Toga AW, Thompson PM (2001) Maps of the brain. Anat Rec 265:37–53
Vickers NJ, Christensen TA, Hildebrand JG (1998) Combinatorial odor discrimination in the brain: attractive and antagonist odor blends are represented in distinct combinations of uniquely identifiable glomeruli. J Comp Neurol 400:35–56
Vickers NJ, Christensen TA, Baker TC, Hildebrand JG (2001) Odour-plume dynamics influence the brain’s olfactory code. Nature 410:466–470
Vosshall LB, Wong AM, Axel R (2000) An olfactory sensory map in the fly brain. Cell 102:147–159
Wang JW, Wong AM, Flores J, Vosshall LB, Axel R (2003) Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell 112:271–282
Wehr M, Laurent G (1996) Odour encoding by temporal sequences of firing in oscillating neural assemblies. Nature 384:162–166
Witthöft W (1967) Absolute Anzahl und Verteilung der Zellen im Hirn der Honigbiene. Z Morphol Tiere 61:160–184
Xu F, Greer CA, Shepherd GM (2000) Odor maps in the olfactory bulb. J Comp Neurol 422:489–495
Zufall F, Leinders-Zufall T, Greer CA (2000) Amplification of odor-induced Ca(2+) transients by store-operated Ca(2+) release and its role in olfactory signal transduction. J Neurophysiol 83:501–512
Acknowledgments
Thanks to Beate Eisermann for help with the figures, Astrid Klawitter for technical assistance, Robert Brandt for help with Amira, Ulrike Schröter for cell tracing, Carsten Duch for help with the electrophysiology, and Mary Wurm for help with the English. Thanks to Randolf Menzel, Silke Sachse and Ulrike Schröter for fruitful comment on the manuscript. The Volkswagenstiftung (I/75-399), the DFG (GRK 120), the BMBF (PTJ0311562X), and HFSP (RGY0050/2001) funded this work.
Animations of the calcium measurements shown in the figures can be accessed under http://galizia.ucr.edu/singleneurons
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Galizia, C.G., Kimmerle, B. Physiological and morphological characterization of honeybee olfactory neurons combining electrophysiology, calcium imaging and confocal microscopy. J Comp Physiol A 190, 21–38 (2004). https://doi.org/10.1007/s00359-003-0469-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-003-0469-0