Abstract
Jasmonates (JAs) play an important role in many developmental processes, such as root growth, leaf senescence, male fertility, and defense responses against insects and pathogens. The F-box protein COI1, which plays a central role in JA signal transduction, perceives the JA signal and is required for all the JA-mediated defense responses against biotic and abiotic stresses. JA signaling elements including COI1 have been extensively investigated in Arabidopsise. However, the elements of the JA signaling pathway in maize are largely unknown. In this study, we identified four F-box protein genes from the maize genome, which share high homology with AtCOI1, designated as ZmCOI1a, ZmCOI1b, ZmCOI1c, and ZmCOI2, collectively ZmCOIs. To test whether or not the homologous genes of maize are functionally conservative in JA signaling, we over-expressed ZmCOIs in the Arabidopsis coi1-1 mutant. The results showed that over-expression of ZmCOI1a, ZmCOI1b or ZmCOI1c in the coi1-1 mutant resulted in the restoration of male fertility, indicating successful complementation of coi1-1 sterility by ZmCOI1a, ZmCOI1b, and ZmCOI1c. However, ZmCOI2 was not able to restore male fertility of the mutant, indicating that ZmCOI2 has a function diverged from JA signaling. Furthermore, over-expression of the ZmCOI1a, ZmCOI1b, and ZmCOI1c genes, except ZmCOI2, which, in the coi1-1 mutant, caused restoration of resistance to the leaf pathogen Botrytis cinerea and the soil-borne pathogen Pythium aristosporum. In addition, a set of JA-dependent genes are highly induced by wounding in the transformants of ZmCOIa, ZmCOI1b, and ZmCOI1c, but not inducible in transformants of ZmCOI2 or in the coi1-1 mutant, indicating that ZmCOIa, ZmCOI1b, and ZmCOI1c, except ZmCOI2, which can compensate coi1-1 mutation of Arabidopsis for the stress defense response. Putting all the data together, our results suggested that ZmCOIa, ZmCOI1b, and ZmCOI1c, but not ZmCOI2, act as AtCOI1 orthologues in maize for JA signal transduction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Jasmonic acid (JA) and derivatives, such as methyl jasmonate (MeJA) and jasmonoyl isoleucine (JA-Ile), are collectively referred to as jasmonates (JAs) (Schaller et al. 2004; Yan et al. 2013). JAs are lipid-derived hormone signals that regulate a wide range of biochemical and physiological processes in plants, ranging from vegetative growth to reproductive development, including seed germination, root growth, trichome development, leaf senescence, tendril coiling, anther dehiscence, pollen viability, fruit ripening, etc (Creelman and Mullet 1997; Yan et al. 2013; Wasternack and Hause 2013; Wasternack 2014; Huang et al. 2017; Zhai et al. 2017; Wasternack and Feussner 2018). In Arabidopsis, JA biosynthesis mutants, such as fad3/7/8 (McConn and Browse 1996), dad1 (Ishiguro et al. 2001), dde2-2 (von Malek et al. 2002) or aos (Park et al. 2002), dde1 (Sanders et al. 2000) or opr3 (Stintzi and Browse 2000) or opr3-3 (Chini et al. 2018), and acx1/5 (Schilmiller et al. 2007), exhibited defects in filament elongation, anther dehiscence, and pollen maturation leading to male sterility. Exogenous application of JA/MeJA on these JA-biosynthetic mutants can restore the stamen development of fad3/7/8 (McConn and Browse 1996), dad1 (Ishiguro et al. 2001), aos (Park et al. 2002), opr3 (Stintzi and Browse 2000) and opr3-3 (Chini et al. 2018), demonstrating that JA is an essential signal for stamen development and pollen maturation in Arabidopsis. Mutants impairing JA signal transduction, such as coi1 (Feys et al. 1994), myb21 (Mandaokar et al. 2006), and myb21myb24 (Mandaokar et al. 2006), are also male-sterile because of reduced filament elongation and lack of anther dehiscence. Over-expression of Jas domain-mutated JAZ protein genes, such as JAZ1Δ3A (Thines et al. 2007), jai3-1 (Chini et al. 2007), JAZ10/JAS1 (Yan et al. 2007), and JAZ10.4 (Chung and Howe 2009), impaired JA signal transduction in the transformants, which exhibited a male-sterile phenotype similar to coi1. In monocotyledonous plants, JA is also an important hormone signal for reproductive growth of flowers and seeds (Yan et al. 2012; Cai et al. 2014). In maize, the JA-deficient mutant opr7opr8 displays a feminized tassel, which consists of pistillate spikelets (female florets) instead of staminate spikelets (male florets) (Yan et al. 2012), indicating that JA is a key signal for sex determination of maize tassels. In rice, the JA biosynthesis mutants hebita and cpm2 (both are the mutants of the single copy AOC gene in rice genome), and the JA signaling mutant Osjar1 showed male sterility due to no anther dehiscence and abnormality of floret architecture (Riemann et al. 2013; Xiao et al. 2014), indicating that JA is required for floret development in rice.
In particular, JAs are a critical hormone signal for plant defense against herbivore insects and pathogens. JA biosynthesis mutants, such as Arabidopsis fad3fad7fad8, aos, opr3 or JA perception mutants jar1, and coi1, as well as those from other plant species, such as tomato jar1 and maize opr7opr8, are highly susceptible to insect attack (McConn et al. 1997; von Malek et al. 2002; Stintzi et al. 2001; Staswick et al. 1998; Xie et al. 1998; Li et al. 2004; Yan et al. 2012). On the other hand, JA-pathway over-expressing mutants, such as cev1, cex1, and fou2, are highly resistant to insect and pathogen attacks (Ellis and Turner 2001; Xu et al. 2001; Bonaventure et al. 2007). Exogenous application of JA or MeJA decreased the suitability of foliage for herbivorous insects in tomato (Thaler et al. 1996; Pauwels et al. 2009). For disease resistance, JA has been demonstrated to be an indispensible signal for resistance/susceptibility to several diseases caused by fungal, bacterial, and viral pathogens (Staswick et al. 1998; Vijayan et al. 1998; Yan and Xie 2015; Wasternack and Strnad 2016; Zhang et al. 2017). The JA perception mutant coi1 displays enhanced susceptibility to the necrotrophic fungi (A) brassicicola, (B) cinerea, P. cucumerina and F. oxysporum (Thomma et al. 1998; Rowe et al. 2010; Thatcher et al. 2009), while the JA biosynthesis mutant aos as well as the signaling mutant coi1 are also highly susceptible to B. cenerea (Rowe et al. 2010). The JA biosynthesis mutants fad3fad7fad8 and jar1 exhibit enhanced susceptibility to the soil-borne pathogen Pythium spp. (Vijayan et al. 1998; Staswick et al. 1998). In maize, the double mutant opr7opr8, deficient in JA biosynthesis, showed extreme susceptibility to Pythium aristosporium (Yan et al. 2012). In addition, JAs have also been reported for their important roles in plant responses to abiotic stresses (Kazan 2015; Per et al. 2018), such as heavy metals (Maksymiec et al. 2005), drought (Brossa et al. 2011), heat stress (Clarke et al. 2009), salt (Zhao et al. 2014) and ozone stresses (Sasaki-Sekimoto et al. 2005).
All the actions of JA are completed in plants by the JA signal transduction machinery, called the JA signaling pathway. The core signaling module of this pathway consists of four major components: a bioactive JA signal, such as JA-Ile, the SCF-type E3 ubiquitin ligase SCFCOI1 complex, jasmonate ZIM-domain (JAZ) repressor proteins, and transcription factors that promote the expression of JA-responsive genes. When JAZ proteins were discovered as the true targets of the SCFCOI1 complex simultaneously by three research groups (Chini et al. 2007; Thines et al. 2007; Yan et al. 2007), the JA signaling model was established: (1) at low intracellular levels of the JA signal, the SCFCOI1 complex (JA receptor) has no E3 ubiquitin ligase activity, resulting in accumulation of JAZ proteins which repress the activity of transcription factors, such as MYC2 that positively regulates JA-responsive genes; and (2) at high levels of the JA signal, such as when a plant is attacked by insects or pathogens, the rapidly accumulated JAs promote SCF(COI1)-mediated ubiquitination of JAZ proteins and subsequently cause them to be degraded via the 26S proteasome. Removal of JAZ proteins causes the release of JAZ-repressed transcription factors, such as MYC2, MYB21, ERF1, etc., and subsequent activation of a number of early JA-responsive genes (Chini et al. 2007, 2016; Thines et al. 2007; Browse and Howe 2008; Katsir et al. 2008; Kazan and Manners 2008; Fonseca et al. 2009; Sheard et al. 2010; Wasternack and Hause 2013; Howe et al. 2018).
Coronatine Insensitive 1 (COI1) is an F-box protein component of the Skp1-Cul-F-box protein (SCF) complex (Devoto et al. 2002) which recruits JAZ proteins and other co-repressor proteins, such as TOPLESS for JA perception and signal transduction (Pauwels et al. 2010). The COI1 gene was identified and cloned from coi1-1, coi1-15, and coi1-18 mutants (Xie et al. 1998), which are insensitive to the bacteria phytotoxin coronatine (a JA analog) and JAs (Feys et al. 1994). Loss-of-function mutants of COI1 in Arabidopsis, such as coi1-1, are completely deficient in all the JA responses (Feys et al. 1994; Xie et al. 1998) due to lack of JA perception. Similar to JA biosynthesis mutants, such as aos (Park et al. 2002), and opr3 (Stintzi and Browse 2000), the JA signaling mutant coi1-1 showed phenotypes of inhibited filament elongation, reduced pollen development and lack of anther dehiscence leading to complete male sterility (Feys et al. 1994; Xie et al. 1998). A number of coi1 alleles have been isolated, and all the knock-out mutants of them, such as coi1-4, coi1-5, coi1-6, coi1-7, coi1-9, coi1-10, etc., share similar phenotypes, such as male-sterile, susceptible to insect damage (He et al. 2012; Huang et al. 2014). To date, the COI1 genes have been identified and characterized from several plant species (Li et al. 2004; Wang et al. 2005, 2014; Peng et al. 2009; Lee et al. 2013) and some of them have been tested experimentally for their function in the JA signaling pathway (Wang et al. 2005; Lee et al. 2013). Arabidopsis has only one copy of the COI1 gene, whereas three AtCOI1 orthologues (OsCOI1a, Os01g0853400; OsCOI1b, Os05g0449500; and OsCOI2, Os03g0265500) have been reported in rice (Yang et al. 2012; Lee et al. 2013). Although maize (Zea mays L.) is an economically important crop in the world, little is known about JA biosynthesis and signaling in this species compared to greater advances in dicot plants, such as Arabidopsis. A study of the opr7opr8 mutant showed that JAs have tremendous roles in a number of developmental and defense processes in maize (Yan et al. 2012). Recently, the lipoxygenase pathway and JA function were reviewed by Borrego and Kolomiets (2016); however, knowledge of JAs and other oxylipins in maize is still limited. In this study, we identified the COI1 orthologues (ZmCOIs) of maize, and their function was analyzed by complementation of the Arabidopsis coi1-1 mutant. Our results indicated that three of four ZmCOIs genes play a crucial role in JA signal transduction in maize.
Materials and Methods
Identification and Phylogenetic Analysis of COI1 Orthologues in Maize and Other Plant Species
To identify the COI1 orthologous genes in maize, we performed a number of blasts against the genome database of maize (https://www.maizegdb.org/) and “Gramene” (http://www.gramene.org/) using the amino acid sequences of Arabidopsis COI1 gene (AtCOI1) and rice COI genes (OsCOI1a, OsCOI1b, and OsCOI2) as the blast queries. To find the COI1 orthologous genes in other plant species, we searched the databases of NCBI (https://www.ncbi.nlm.nih.gov/), PlantGDB (http://plantgdb.org/cgi-bin/blast/PlantGDBblast) using the sequences of reported COI1 genes, such as Arabidopsis COI1 (Xie et al. 1998), tomato COI1 (Li et al. 2004), OsCOIs (Lee et al. 2013), GmCOI1 (Wang et al. 2005), HbCOI1 (Peng et al. 2009) and AsCOI1 (Liao et al. 2015) as search queries. Multiple sequence alignment and phylogenetic analysis was performed by MEGA5.0 software.
Plant Material and Growth Conditions
The 3rd leaf of maize inbred line B73 was used to extract genomic DNA or total RNA, which were used to amplify genomic and cDNA sequences of ZmCOIs. The cDNA sequences were used for construction of over-expression vectors. Arabidopsis coi1-1 heterozygous and wild-type (columbia-0) seeds were kindly provided by Dr. Daoxin Xie (Tsinghua University, Beijing, China).
Maize seeds were planted in 2-L pots filled with mixed soil (vermiculite: organic substrate: loam = 1:1:1). Maize seedlings were grown in the greenhouse, with controlled conditions (temperature was controlled at 25–35 °C). Arabidopsis seeds were surface-sterilized, and planted in 0.3-L pots with artificial mixed soil (vermiculite: organic substrate: peat: perlite = 3:3:3:1). The pots were placed under 16 h day and 8 h night cycles at 22 °C in a growth chamber.
Construction of Over-Expression Vectors of ZmCOIs
The full-length coding region of the ZmCOIs was PCR-amplified from the maize B73 cDNA with gene-specific primers. The PCR products were cloned into the pEASY-Blunt Zero vector (Transgen Biotech, pEASY-Blunt Zero cloning kit) for sequencing. The sequencing-verified sequences of ZmCOIs were used for over-expression vector construction. The verified clones of ZmCOIs were subsequently cloned into the pANIC6E vector (Mann et al. 2012) by gateway cloning techniques using BP Clonase™ Enzyme Mix (Invitrogen, Catalog no. 11789-013) and LR Clonase™ Enzyme Mix (Invitrogen, Catalog no. 11789-019). The construction procedure was performed according to the manufacturer’s instructions for the cloning kits. In the final over-expression construction, the gene of interest was controlled under a ZmUbi1 promoter. The final constructions (pANIC6E-ZmCOI1a, -ZmCOI1b, -ZmCOIc, -ZmCOI2) were transformed into Agrobacteria strain GV3101 that was used to transform Arabidopsis.
Selection of coi1-1 Homozygote Transformants
coi1-1 heterozygous plants were used to transform with the constructions of ZmCOIs. The coi1-1 homozygous segregants were selected by PCR genotyping using primers P1&P2 and restriction enzyme Xcm I (Xie et al. 1998).
Gene Expression by qPCR and RT-PCR
To test the ZmCOIs expression level in the tissues of maize plant, total RNA was extracted from tissues including ear leaf, young cob, young tassel, brace roots, internode, silk, etc. To test the effect of hormones on the gene expression of ZmCOIs, the V3-stage-seedlings of B73 were sprayed with 100 µM of JA, ABA, ACC, NAA, and GA3 and 2.5 mM of SA solution containing 0.1% Tween-20. The 3rd leaves of the treated plants were harvested at the time points of 0, 6, 12, 24, 48, and 72 h after treatment and frozen in liquid nitrogen for further use to isolate RNA. To test the gene expression of JA-dependent genes in over-expression transformants, the leaves of WT, coi1-1 and transformants were mechanically wounded and samples harvested at 0, 0.5, 1.5, and 6 h after wounding for further RNA extraction.
The total RNA of all the samples was extracted using TRIzol™ Reagent (Thermo Fisher Scientific, Catalog No. 15596018). The first strand of cDNA was synthesized using EasyScript first-strand cDNA synthesis super mix (Transgen Biotech, Catalog No. AE301-02). The gene expression of ZmCOIs was detected by real-time quantitative PCR (qPCR) using PowerUp™ SYBR™ Green Master Mix (Thermo Fisher Scientific, Catalog No. A25741). The maize EIF4α gene was used as the reference gene of qPCR. In the transgenic Arabidopsis plants, JA-dependent gene expression in response to mechanical wounding was detected by semi-quantitative RT-PCR according to Marone et al. (2001) using the β-actin2 gene as the reference gene.
Pathogen Inoculation of ZmCOIs-Over-Expressed Plants
To test the defense response of transgenic plants, two pathogen species (the necrotrophic pathogen Botrytis cinerea and the soilborne root pathogen Pythium aristosporum) were applied to Arabidopsis WT, coi1-1 and over-expressed transformants of ZmCOIa, ZmCOI1b, and ZmCOI1c in the coi1-1 mutant.
For the Botrytis cinerea culture and inoculation, purified B. cinerea was obtained from Chunhao Jiang (Nanjing Agricultural University, Nanjing, China). A piece of B. cinerea culture was transferred to a PDA (potato dextrose agar) plate grown at 25 °C for 10–15 days under a fluorescence light of < 1000 lx illumination. The conidia were collected and suspended in sterile water containing 0.025% Tween-20. The whole plants were inoculated by spraying a spore suspension (5 × 105 spores /ml) until the leaves were fully covered by the suspension. Inoculated plants were kept at 25 °C in a box covered with a piece of plastic membrane for 1 day and then they were put back in the growth chamber for 3–5 days until the typical symptom appeared.
For the Pythium aristosporum culture and inoculation, P. aristosporum was isolated from the rotted roots of the maize JA biosynthesis mutant opr7opr8 (Yan et al. 2012). The isolate was inoculated to a carrot–agar medium plate and grown at room temperature with 200–1000 lx illumination until oospores formed in the medium. The medium culture was blended with sterile water and passed through two layers of gauze. The denseness of suspension was adjusted with sterile water to a concentration of 105 oospores/ml, which was counted under a microscope using a hemocytometer. The plants were inoculated by adding the suspension to the soil to infect the roots. Each plant received 10 ml of the Pythium suspension. The inoculated plants were kept at 25 °C with illumination at about 1000 lx. At 3–5 days, photographs for the symptoms of disease were taken.
Wound Treatment
Mechanical wound treatment was conducted as described by Reymond et al. (2000). The samples were harvested at 0, 0.5, 1.5, and 6 h after wounding.
Results
Identification of the COI1 Orthologues in the Maize Genome
The COI1 gene has been identified from the Arabidopsis mutant coi1-1, coi1-15 and coi1-18 (Xie et al. 1998) and the genome has only a single COI1 gene copy (Lee et al. 2013). Three COI1 orthologues in rice, OsCOI1a (Os01g0853400), OsCOI1b (Os05g0449500), and OsCOI2 (Os03g0265500), have been reported (Lee et al. 2013). To identify the COI1 orthologous genes in maize, we searched the maize genome by performing blasts against the genome database of maize (https://www.maizegdb.org/) and “Gramene” (http://www.gramene.org/) using the amino acid sequences of the Arabidopsis COI1 gene (AtCOI1) and rice COI genes (OsCOI1a, OsCOI1b, and OsCOI2) as the blast queries and four COI1 orthologous genes in maize genome have been identified: GRMZM2G125411, GRMZM2G151536, GRMZM2G353209, and GRMZM2G079112, designated as ZmCOI1a, ZmCOI1b, ZmCOI1c, ZmCOI2 respectively, collectively called ZmCOIs.
The amino acid (AA) sequence alignment of ZmCOIs with AtCOI1 showed that ZmCOIs share 54.6–57.2% AA identity with AtCOI1 (Fig. 1; Fig. S1), indicating that ZmCOIs share a low homology with AtCOI1. However, the protein domains ZmCOIs and AtCOI1 share conservative F-box and leucine-rich repeats (LRRs) (Fig. 1), indicating that they may share a similar molecular function. The COI1 protein is a critical component of the SCF(COI1)-JAZ complex, the core machinery of JA perception in plants (Katsir et al. 2008; Sheard et al. 2010). The COI1 protein possesses 16 key amino acid residues (asterisks or triangles in Fig. 1), which are supposed to be the binding sites of JA-Ile or JAZ proteins with COI1 (Yan et al. 2009; Sheard et al. 2010). We found that ZmCOI1a, ZmCOI1b, and ZmCOI1c share 13–14 conservative key amino acid residues with AtCOI1 (Fig. 1), but ZmCOI2 has two additional divergent points of these key amino acids (solid boxes in Fig. 1), suggesting ZmCOI1a, ZmCOI1b, and ZmCOI1c may have a conservative molecular function as AtCOI1, but ZmCOI2 might have evolved into a new divergent function category.
Amino acid sequence alignment of AtCOI1 and ZmCOIs. Deduced amino acid sequences of AtCOI1 and ZmCOIs were aligned using the DNAMAN7.0 program. Black shaded letters indicate identical residues. ZmCOIs and AtCOI1 share conservative F-box and Leucine-rich repeats (LRRs). Asterisks indicate the binding sites of coronatine/JA-Ile in the COI1-JAZcomplex (Yan et al. 2009; Sheard et al. 2010). Triangles indicate JAZ-binding sites involved in the COI-JAZ interaction (Sheard et al. 2010). Solid boxes indicate the amino acid residues in ZmCOI2 which are divergent from AtCOI1 and ZmCOI1a/b/c
The AA sequence alignment of ZmCOIs showed that the members of the ZmCOIs family share higher AA identity with each other. The AA identity is 78.4% between ZmCOI1a and ZmCOI1b, 78.9% between ZmCOI1a and ZmCOI1c or 93.5% between ZmCOI1b and ZmCOI1c (Fig. S2). The AA identities of ZmCOI2 with ZmCOI1a, ZmCOI1b, and ZmCOI1c are 60.9%, 61.6% and 60.1%, respectively (Fig. S2), indicating that ZmCOI2 is a highly different gene from ZmCOI1a, ZmCOI1b, and ZmCOI1c in the maize genome. We have also blasted “Gramene” using the amino acid sequences of ZmCOIs as the blast queries and identified the COI1 orthologous genes in purple false brome Brachpodium distachyon, millet (Setaria italica), and sorghum (Sorghum bicolor). Rice, Brachpodium, millet, and sorghum all have three COI1 orthologous genes in their genomes (Fig. S3). AA sequence analysis showed that ZmCOIs are highly similar to the COI1 orthologous genes in rice (OsCOIs), Brachpodium (BdCOIs), millet (SiCOIs), and sorghum (SbCOIs) (Fig. S3), indicating that COI1 genes are highly conserved in cereals. ZmCOIs and SbCOIs have AA identity of more than 93% (Fig. S3), indicating that sorghum is a highly close species to maize for the genome evolution.
Phylogenetic Analysis of COI1 Orthologous Genes in Plants
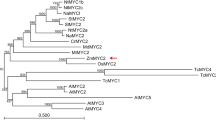
The COI1 protein, a critical component of the JA-perception complex (Katsir et al. 2008; Sheard et al. 2010), must be an essential protein for all higher plants, in which it acts as an indispensable growth regulator for varied developmental processes and defense responses (Yan et al. 2013). To determine the evolutionary relationship among COI1 proteins in plants, the AA sequences of COI1 orthologues from 36 plant species were obtained by searching the NCBI database (https://www.ncbi.nlm.nih.gov/) and “Gramene”, using AtCOI1, OsCOIs and ZmCOIs as the searching queries and an unrooted phylogenetic tree of these COI1s was constructed with maximum likelihood (ML) algorithms by the software MEGA5.0 (Fig. 2). This phylogenetic tree displayed that all the COI1 proteins we applied here clustered into two clades: dicots and monocots (Fig. 2), indicating that dicotyledonous and monocotyledonous COI1 genes have their own ancestral lineage. The dicotyledonous clade can be divided into five groups (I–V) and AtCOI1 belongs to group II. The monocotyledonous COI1 proteins clustered into two groups (VI–VII) (Fig. 2). Group VI contains COI1a and COI1b and maize COI1c. Group VII includes COI2 of the monocotyledonous species. On this tree, we also noticed that maize COIs are closer to sorghum COIs than other cereals, revealing a close phylogenetic relationship between these two species.
Tissue-Specific Expression of ZmCOIs Genes and Their Responses to Hormone Treatments
The maize genome contains four COI1 orthologous genes (Fig. 1), but some of them may not be expressed. To know which of the ZmCOIs are expressed, we analyzed the expression levels of ZmCOIs in the leaf, internode, brace roots, ear cob, young tassel and silk using the quantitative RT-PCR technique. The results showed that all the members of ZmCOIs are constitutively expressed in all the tissues tested (Fig. S4), among them ZmCOI1a and ZmCOI1b are highly-expressed genes whereas ZmCOI1c and ZmCOI2 are less expressed, indicating that maize may largely depend on the function of ZmCOI1a and ZmCOI1b for JA signal transduction. To further verify our results, we downloaded the data of RNA-Seq expression from MaizeGDB (https://www.maizegdb.org/), and the results confirmed that ZmCOI1a and ZmCOI1b are highly-expressed and ZmCOI1c and ZmCOI2 less-expressed genes in 79 tissues of maize plants (Fig. S5). Interestingly, these data showed that ZmCOI2 is highly expressed in anther and in seed endosperm (Fig. S6), indicating that ZmCOI2 may be involved in anther or seed development.
The COI1 gene is a critical gene for JA signal transduction (Feys et al. 1994; Xie et al. 1998). To understand if the expression of ZmCOIs responds to plant hormones, the transcript level of ZmCOIs was analyzed by quantitative RT-PCR in the leaves sprayed with JA, ABA, ACC, GA3, NAA, and SA (see “Materials and Methods”). The results showed that ZmCOI1a and ZmCOI1b genes were strongly induced by JA and ABA (Fig. 3), but there was no significant induction by ACC, GA3, NAA, and SA (Fig. 3). For JA treatment, the expression of ZmCOIa and ZmCOI1b was increased more than 15 times in 12 h after treatment (Fig. 3). For ABA treatment, ZmCOIa and ZmCOI1b increased more than 8 and 5 times, respectively, in 12 h after treatment (Fig. 3). This result indicates that the expression level of ZmCOI1a and ZmCOI1b may be regulated by endogenous JA and ABA. ZmCOI1c and ZmCOI2 genes are less-expressed genes in maize tissues (Fig. 3) and they are just slightly induced by JA and ABA, but not induced by ACC, GA3, NAA, and SA (Fig. 3), suggesting that ZmCOI1c and ZmCOI2 are low-expressed but constitutively expressed genes in maize.
The expression analysis of ZmCOIs in the leaf in response to hormone treatments. The hormone solutions of a jasmonic acid (JA, 100 µM), b abscisic acid (ABA, 100 µM), c 1-aminocyclopropane-1-carboxylic acid (ACC, 100 µM), d gibberellic acid3 (GA3, 100 µM), e 1-naphthylacetic acid (NAA, 100 µM). f Salicylic acid (SA, 2.5 mM) was sprayed to B73 seedlings at the V3 stage. The expression levels were detected by quantitative RT-PCR. Three biological replicates were performed. EIF4α was used as a reference gene. Error bars indicate the standard deviations (SD) of the mean value of three biological replicates. Different letters represent means statistically different (p < 0.05)
Over-Expression of ZmCOI1a/b/c in Arabidopsis coi1-1 Mutant Restores Male Fertility of the Mutant
To efficiently test whether the ZmCOIs are functionally similar to Arabidopsis COI1, we transformed each of the ZmCOIs into the Arabidopsis coi1-1 mutant to test whether ZmCOI1a, ZmCOI1b, ZmCOI1c, and ZmCOI2 can complement the JA-insensitivity-related phenotypes of coi1-1. Because coi1-1 homozygous is male-sterile and is not suitable to be transformed by floral dip (Clough and Bent 1998), we transformed each ZmCOIs gene into coi1-1 heterozygous plants. The transgenes existence in the genome of transformant plants (T1) was confirmed by PCR amplification using ZmCOIs-specific primers (Fig. S10), and the mRNA of the transgenes was detected by RT-PCR amplification using ZmCOIs-specific primers (Fig. S10). In the segregation generation (T1) of transformants, we selected only coi1-1 homozygous transformants containing a transgene gene (ZmCOI1a/ZmCOI1b/ZmCOI1c/ZmCOI2) by PCR genotyping (see “Materials and Methods”). A large portion (15.6–36.3%) of transgenic coi1-1 mutant plants transformed with ZmCOI1a or ZmCOI1b or ZmCOI1c showed fertile flowers (Table 1; Fig. 4) and good seed-bearing (Table 1), whereas transgenic plants with ZmCOI2 were male-sterile and non-seed-bearing (Table 1; Fig. 4). These results indicated that ZmCOI1a, ZmCOI1b, and ZmCOI1c are capable of complementing the Arabidopsis coi1-1 mutant but ZmCOI2 is not, suggesting that ZmCOI1a, ZmCOI1b, and ZmCOI1c have similar functions as the Arabidopsis COI1 gene whereas ZmCOI2 has a divergent function from COI1. All the T2 generation plants of the ZmCOI1a, ZmCOI1b, and ZmCOI1c T1 generation (ZmCOI1a, ZmCOI1b, and ZmCOI1c transformants) showed fertility and seed-bearing (seeds/silique) similar to T1 plants (Table 1).
The flower morphological phenotypes of coi1-1 homozygous mutants transformed with ZmCOI1a, Zm-COI1b, ZmCOI1c, and ZmCOI2, respectively, at the T1 generation. a Inflorescences of 8-week-old plants of wild-type (WT) and transformants. b The flowers of 6-week-old plants of WT and transformants. c Fully developed siliques of WT and transformants. d The seeds in a siliques of WT and transformants
Over-Expression of ZmCOI1a/b/c in coi1-1 Mutant Causes Immunity Recovery Against Necrotrophic Pathogen Botrytis cinerea and Oomycete Pathogen Pythium aristosporum
JA is one of the major defense hormones in plants (Browse 2009). The Arabidopsis JA biosynthesis mutant aos and JA signaling mutant coi1-1 are highly susceptible to the necrotrophic pathogen Botrytis cinerea (Rowe et al. 2010). In this study, we tested whether ZmCOIs are able to complement the coi1-1 mutant for its defense ability against B. cinerea. The transgenic plants (homozygous coi1-1 transformed with one of ZmCOIs) as well as WT were inoculated with B. cinerea spore suspension. The results showed that the transgenic plants of ZmCOI1a, ZmCOI1b, and ZmCOI1c are highly resistant to B. cinerea as WT (Fig. 5), but the transgenic plants of ZmCOI2 remained susceptible to B. cinerea just like the coi1-1 mutant (Fig. 5), indicating that the transgene ZmCOI1a, or ZmCOI1b, or ZmCOI1c in transformants, but not ZmCOI2, imitates AtCOI1 in the COI1-mediated defense response against necropathogens.
The susceptibility of coi1-1 transformants with ZmCOI1a, Zm-COI1b, ZmCOI1c, and ZmCOI2, respectively, to Botrytis cenerea and Pythium aristospor. a The 4-week-old plants of genotypes indicated were sprayed with spore suspension of Botrytis cenerea. The picture was taken at 72 h after inoculation. b Four-week-old plants of genotypes indicated were inoculated at the root system with a suspension of Pythium aristosporum. The pictures were taken at 72 h after inoculation
Pythium spp. are soilborne pathogens and are able to infect a wide variety of plants. The Arabidopsis triple mutant fad3 fad7 fad8 cannot accumulate jasmonate and is extremely susceptible to root rot caused by the fungal root pathogen Pythium mastophorum (Vijayan et al. 1998). The Arabidopsis JA signaling mutant jar1-1 is highly susceptible to a soil oomycete Pythium irregular (Staswick et al. 1998). To further demonstrate the function of ZmCOIs for defense response, we inoculated the transgenic plants with the suspension of the root pathogen Pythium aristosporum by dropping the suspension to the soil of each plant. Our results showed that the Arabidopsis coi1-1 mutant is highly susceptible to Pythium aristosporum (Fig. 5) and WT is resistant to this pathogen. Over-expression of ZmCOI1a, ZmCOI1b, and ZmCOI1c in the coi1-1 mutant provided resistance to the coi1-1 mutant and ZmCOI2 is not able to compensate the susceptibility to P. aristosporum, indicating again that ZmCOI1a, ZmCOI1b, and ZmCOI1c but not ZmCOI2 are functional orthologues of AtCOI1 in COI1-mediated defense against the soilborne pathogen Pythium spp.
Over-Expression of ZmCOI1a/b/c in Arabidopsis coi1-1 Mutant Restores the Wound Response of JA-Dependent Genes
The COI1 gene is required for expression of all the JA- or wound-inducible genes in Arabidopsis (Devoto et al. 2005). In this study, we tested the expression of a number of JA-dependent genes in ZmCOIs transformants. The results showed that the eight typical COI1-dependent genes chosen here are not inducible by wounding in the coi1-1 mutant, but induced in WT (Fig. 6). All the eight genes are highly induced by wounding in the transformants of ZmCOIa, ZmCOI1b, and ZmCOI1c in the coi1-1 background, but not in the coi1-1/ZmCOI2 (Fig. 6), indicating that ZmCOIa, ZmCOI1b, and ZmCOI1c but not ZmCOI2 can compensate the coi1-1 mutation in Arabidopsis for the wound-induced defense response. These gene expression results supported that ZmCOIa, ZmCOI1b, and ZmCOI1c are the functional orthologous genes in maize to AtCOI1 in Arabidopsis and ZmCOI2 may not have the typical function of the SCFCOI1 complex genes in defense and development processes.
The expression of a set of JA-dependent genes in coi1-1 transformants with ZmCOI1a, Zm-COI1b, ZmCOI1c, and ZmCOI2, respectivel,y were detected by RT-PCR upon wounding. The leaves of the plants of the genotypes indicated were wounded with forceps and the mRNA levels of eight JA-dependent genes (AOS, OPR3, JAZ1, JAZ3, JAZ10, JAL23, PDF1.2a, and VSP1) were detected after wounding at the time points indicated. The accession numbers of the eight genes are listed in Table S1
Discussion
Identification of ZmCOIs and Orthologues in Other Monocots and Comparison Analysis with AtCOI1
In this study, we isolated and characterized the COI1 orthologous genes in maize. Using the nucleotide sequence and amino acid (AA) sequence of the Arabidopsis COI1 gene (AtCOI1) and rice COI1 orthologues (OsCOI1a, OsCOI1b, and OsCOI2) we blasted the maize genome (http://www.MaizeGDB.org) and found four COI1 orthologous genes in the maize genome. According to the nomenclature used in rice COI1 orthologues (Lee et al. 2013), we designated the COI1 orthologues of maize as ZmCOI1a, ZmCOI1b, ZmCOI1c, and ZmCOI2. This designation means that the first three of the four genes are the closest orthologues of AtCOI1, and the last one (i.e. ZmCOI2) is a less similar orthologue with AtCOI1 than the first three but is still highly homologous to AtCOI1. To understand better why Arabidopsis just has one COI1 gene but monocots, such as rice and maize, have many copies of COI1, we have carried out two analyses in this study: (1) how many copies of COI1 orthologues are in other monocots? And (2) what is the homology among all the COI1 genes in monocotyledonous and dicotyledonous plants? Using the AA sequences of ZmCOI1s and OsCOI1s, we blasted the genomes in the database Gramene (http://www.gramene.org/), and three COI1 genes were found in Brachypodium distachyon, Setaria italic, and Sorghum bicolor, respectively. Using similar nomenclature to OsCOIs or ZmCOIs, we designated them as BdCOI1a, BdCOI1b and BdCOI2 for the Brachypodium, SiCOI1a, SiCOI1b and SiCOI2 for foxtail millet and SbCOI1a, SbCOI1b, and SbCOI2 for sorghum. Amino acid sequence alignment showed that AtCOI1 shares 53.9–57.6% AA identity with the COI1 orthologues in the five monocotyledonous species (Fig. S1), suggesting that, in the cereals, COI1 orthologues are substantially different from AtCOI1. Furthermore, we analyzed COI1 orthologues in the five monocots. The alignment showed that ZmCOI1s share more than 78% AA identity with OsCOI1s, BdCOI1s, SiCOI1s and SbCOI1s (Fig. S3). These results indicated that COI1 genes in monocots are highly conservative. Especially, the COI1 genes in maize and sorghum are so close to each other that they share more than 93% AA identity (Fig. S3).
Phylogenetic Analysis and Evolution of COI1 Genes in Plants
To understand the evolutionary relationship of COI1 genes in plants, the sequences of the COI1 orthologues in 36 plant species were searched and identified from a number of genomic databases (Gramene, PlantGDB, NCBI, Phytozome, Rice Genome Annotation Project, MaizeGDB). Phylogenetic analysis of these COI1 orthologues showed that all the AA sequences fall into two separate clades (Fig. 2). The first clade contains all the COI1 genes from dicotyledonous species and the second clade from monocotyledonous plants (Fig. 2). Placing the AA identity of COI1s between maize and other species into plant evolutionary lineages (Fig. S9), we found that COI1 is a good marker for speciation of the evolutionary lineage. If the divergence time is earlier, the AA identity of COI1s between maize and a species is higher, and vice versa. For example, the divergence between maize and sorghum happened 13 Mya and the AA identity between them is about 94%, whereas between maize and Brachpodium is 48 Mya and the AA identity about 80% (Fig. S9). Monocotyledonous plants diverged from dicotyledonous at 140–150 Mya (Chaw et al. 2004) and the AA identity between maize and Arabidopsis is about 55% (Fig. S9).
In the phylogenetic analysis, we noticed that dicots have only one COI1 gene but that monocots have 3 or more. This phenomenon may not be a divergence feature between dicots and monocots, and is just because of the limited sequences of COI1 genes in dicots. Arabidopsis (2n = 10, diploid) has a single copy of COI1 (Lee et al. 2013). Tomato (Solanum lycopersicon, 2n = 24, diploid) also has a JAI1 gene (COI1 orthologue in tomato) (Li et al. 2004). Using JAI1 and AtCOI1 to blast the tomato genome in Gramene, we get only one gene, Solyc05g052620.2, which is a 100% match to JAI1, suggesting the tomato genome just has a single copy of the COI1 gene. Wild tobacco (Nicotiana attenuate, 2n = 24, diploid) was found to have one COI1 gene by Southern blot analysis (Paschold et al. 2007). A COI1 gene has been isolated from cultivated tobacco (Nicotiana tabacum, 2n = 4x = 48, allotetraploid) (Shoji et al. 2008). Using AtCOI and JAI1 as the query to blast the tobacco genome, we can obtain four loci orthologous to AtCOI1 or JAI1, indicating that there are four COI1 genes existing in the tobacco genome. For soybean (Glycine max, 2n = 40, paleotetraploid), there is a COI1 gene (Wang et al. 2005). Searching the soybean genome in Gramene, we found four COI1-like genes (GLYMA11G34940, GLYMA18G03420, GLYMA14G06740, GLYMA02G42150) existing in the soybean genome. In Brassica napus (2n = 4x = 38, allotetraploid), there are 8 COI1-like genes (Wang et al. 2015). Putting all the information together, we can conclude that the diploid dicots have a single copy of the COI1 gene, whereas the polyploid or paleopolyploid dicots may possess a number of COI1 orthologues in their genomes. We also noticed that the monocotyledonous species applied here have three or more COI1 orthologues. This must be because of the species evolution of the monocots. A number of studies for species evolution have concluded that all grass genomes are derived from a shared paleopolyploid ancestor (n = 12) (Eckardt 2008; Devos 2010; Zhang et al. 2012), which underwent further whole genome duplication events and nested chromosome fusion events to form the genomes of the cereals such Brachypodium, rice, maize, sorghum, wheat, etc. (Zhang et al. 2012). This means that the modern cereals must have the features of paleopolyploids, indicating that most genes in these genomes are duplicated or multiple-copied. Our data suggest that the paleopolypoidy of Brachypodium, rice, maize, sorghum and millet is the reason that they comprise several copies of COI1 orthologues in their genomes.
The Conservative Function of ZmCOI Orthologues
Our major concern in this study is the function of the ZmCOI1s. Maize possesses four orthologues of AtCOI1. In this study, we applied the “mutant complementation” approach to characterize the function of the ZmCOI1 genes. This approach has been successfully used for functional analysis of GmCOI1 (Wang et al. 2005) and OsCOI1s (Lee et al. 2013). Here, we used this approach to carry out the functional analysis of ZmCOI1s by overexpression of a single ZmCOI1 orthologue into the Arabidopsis mutant coi1-1, in which the AtCOI1 function was completely lost (Xie et al. 1998). The Arabidopsis mutant coi1-1 is impaired in JA responses, resulting in male sterility of flowers and insensitivity of roots and shoots to JA/MeJA treatment (Xie et al. 1998). Our results showed that coi1-1 transformants with overexpressed ZmCOI1a, ZmCOI1b or ZmCOI1c are male-fertile but transformants with ZmCOI2 remain male-sterile (Fig. 4), indicating that ZmCOI1a, ZmCOI1b, and ZmCOI1c have a similar function as AtCOI1, while ZmCOI2 may not be a functional COI1 orthologue in maize. Furthermore, necrotrophic pathogen assays and caterpillar feeding assays showed that coi1-1 transformants with overexpressed ZmCOI1a, ZmCOI1b or ZmCOI1c have higher resistance to the pathogen fungi and chewing insect Spodoptera exigua than coi1-1 mutants, but coi1-1 transformants with ZmCOI2 are just like coi1-1 mutants, which are highly susceptible to the pathogen B. cinerea or the insect S. exigua (Fig. S7). These results further indicated that ZmCOI1a, ZmCOI1b, and ZmCOI1c function similar to AtCOI1 for JA signaling, but ZmCOI2 does not show the function of AtCOI1 in Arabidopsis. ZmCOI1a, ZmCOI1b, ZmCOI1c, and ZmCOI2 shared about 55% AA identity with AtCOI1. Interestingly, ZmCOI2 has just 1.8% less AA identity than ZmCOI1b or ZmCOI1c and it cannot complement the Arabidopsis coi1-1 mutant. Our results are in accordance with results from a OsCOI1s study (Lee et al. 2013), which showed overexpression of either OsCOI1a or OsCOI1b in the Arabidopsis coi1-1 mutant resulting in the restoration of JA signal transduction and successful complementation of the coi1-1 mutation phenotypes, but OsCOI2 was not able to successfully complement the coi1-1 mutant, although the OsCOI2 protein interacted with a few OsJAZs (Lee et al. 2013). Lee et al. (2013) showed that the H391 of the OsCOI2 protein is a crucial site of OsCOI2 function divergence. They further showed that a single mutation that replaced the conserved His (H)-391 residue of the OsCOI2 protein with a Tyr (Y)-391 enabled OsCOI2 to complement the coi1-1 mutant (Lee et al. 2013). Similarly, our results showed that ZmCOI2 cannot complement the phenotypes of the coi1-1 mutant. However, “H391”does not exist in ZmCOI2 (the corresponding site of H391 in ZmCOI2 is Y, not H) (Fig. S8). And the corresponding site of “H391” in ZmCOI1a, ZmCOI1b, and ZmCOI1c are Y, not H (Fig. S8). Therefore, the hypothesis that H391 of OsCOI2 significantly changed the protein function does not applied to ZmCOI2. For ZmCOI2 function, we have a similar hypothesis that a single amino acid substitution can significantly change the protein physiological function. With this hypothesis, we analyzed the ZmCOI2 AA sequence with all the coi1 alleles in Arabidopsis which have been reported (He et al. 2012; Huang et al. 2014). This analysis showed that none of the AA changes in the coi1 alleles is equal to the amino acid substitution in ZmCOI2 in comparison to ZmCOI1a, ZmCOI1b, and ZmCOI1c. However, the coi1-7 site may be important for ZmCOI2. The Arabidopsis coi1-7 allele is a loss-of-function mutant caused by an amino acid substitution: G155E. The corresponding site of coi1-7 in the ZmCOI2 or OsCOI2 protein is S instead G (Fig. S8), indicating that, if ZmCOI2 evolved a divergent function rather than mediating JA signaling, this S may be an important amino acid residue which might significantly change the function of ZmCOI2. Noticeably, further studies are needed to elucidate the biological function divergence of ZmCOI2 as well as other COI2 genes in cereals.
In conclusion, four ZmCOIs were identified from the maize genome and their functional features have been characterized in this study. JA is an essential phytohormone that controls many aspects of plant development and defense in response to endogenous developmental cues and environmental stimuli. COI1s are one of the critical components for JA signal perception in response to diverse stimuli. Our results provided evidence that COI1 genes may be functionally conserved in monocotyledonous and dicotyledonous plants, suggesting that COI1 genes from different plant species share similar basic functions for JA signal transduction. In addition, it is possible that some orthologues of plant COI1 genes, such as ZmCOI2 and OsCOI2, may have evolutionarily diverged and gained new functions for other signal transductions rather than JA signaling.
Accession Numbers of Genes
The ZmCOI1a, ZmCOI1b, ZmCOI1c, and ZmCOI2 genes can be found in the dababase of http://www.maizeGDB.org or http://www.gramene.org by their B73_RefGen_v3 (or v4) version ID numbers: ZmCOI1a, GRMZM2G125411 (or Zm00001d042833); ZmCOI1b, GRMZM2G151536 (or Zm00001d010082), ZmCOI1c, GRMZM2G353209 (or Zm00001d038273), and ZmCOI2, GRMZM2G079112 (or Zm00001d028543). The accession numbers of other genes in Genbank used in this study were listed in Table S1.
References
Bonaventure G, Gfeller A, Rodríguez VM, Armand F, Farmer EE (2007) The fou2 gain-of-function allele and the wild-type allele of Two Pore Channel 1 contribute to different extents or by different mechanisms to defense gene expression in Arabidopsis. Plant Cell Physiol 48:1775–1789
Borrego EJ, Kolomiets MV (2016) Synthesis and functions of jasmonates in maize. Plants (Basel) 5:E41
Brossa R, López-Carbonell M, Jubany-Marí T, Alegre L (2011) Interplay between abscisic acid and jasmonic acid and its role in water-oxidative stress in wild-type, ABA-deficient, JA-deficient, and ascorbate-deficient Arabidopsis plants. J Plant Growth Regul 30:322–333
Browse J (2009) Jasmonate passes: muster a receptor and targets for the defense hormone. Annu Rev Plant Biol 60:183–205
Browse J, Howe GA (2008) New weapons and a rapid response against insect attack. Plant Physiol 146:832–838
Cai Q, Yuan Z, Chen M, Yin C, Luo Z, Zhao X, Liang W, Hu J, Zhang D (2014) Jasmonic acid regulates spikelet development in rice. Nat Commun 5:3476
Chaw SM, Chang CC, Chen HL, Li WH (2004) Dating the monocot-dicot divergence and the origin of core eudicots using whole chloroplast genomes. J Mol Evol 58: 424–441
Chini A, Fonseca S, Fernández G, Adie B, Chico JM, Lorenzo O, García-Casado G, López-Vidriero I, Lozano FM, Ponce MR, Micol JL, Solano R (2007) The JAZ family of repressors is the missing link in jasmonate signalling. Nature 448:666–671
Chini A, Gimenez-Ibanez S, Goossens A, Solano R (2016) Redundancy and specificity in jasmonate signalling. Curr Opin Plant Biol 33:147–156
Chini A, Monte I, Zamarreño AM, Hamberg M, Lassueur S, Reymond P, Weiss S, Stintzi A, Schaller A, Porzel A, García-Mina JM, Solano R (2018) An OPR3-independent pathway uses 4,5-didehydrojasmonate for jasmonate synthesis. Nat Chem Biol 14:171–178
Chung HS, Howe GA (2009) A critical role for the TIFY motif in repression of jasmonate signaling by a stabilized splice variant of the JASMONATE ZIM-domain protein JAZ10 in Arabidopsis. Plant Cell 21:131–145
Clarke SM, Cristescu SM, Miersch O, Harren FJ, Wasternack C, Mur LA (2009) Jasmonates act with salicylic acid to confer basal thermotolerance in Arabidopsis thaliana. New Phytol 182:175–187
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Creelman RA, Mullet JE (1997) Biosynthesis and action of jasmonates in plants. Annu Rev Plant Biol 48:355–381
Devos KM (2010) Grass genome organization and evolution. Curr Opin Plant Biol 13:139–145
Devoto A, Nieto-Rostro M, Xie D, Ellis C, Harmston R, Patrick E, Davis J, Sherratt L, Coleman M, Turner JG (2002) COI1 links jasmonate signalling and fertility to the SCF ubiquitin-ligase complex in Arabidopsis. Plant J 32:457–466
Devoto A, Ellis C, Magusin A, Chang HS, Chilcott C, Zhu T, Turner JG (2005) Expression profiling reveals COI1 to be a key regulator of genes involved in wound- and methyl jasmonate-induced secondary metabolism, defence, and hormone interactions. Plant Mol Biol 58:497–513
Eckardt NA (2008) Grass genome evolution. Plant Cell 20:3–4
Ellis C, Turner JG (2001) The Arabidopsis mutant cev1 has constitutively active jasmonate and ethylene signal pathways and enhanced resistance to pathogens. Plant Cell 13:1025–1033
Feys B, Benedetti CE, Penfold CN, Turner JG (1994) Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male-sterile, insensitive to methyl jasmonate, and resistant to a bacterial pathogen. Plant Cell 6:751–759
Fonseca S, Chico JM, Solano R (2009) The jasmonate pathway: the ligand, the receptor and the core signalling module. Curr Opin Plant Biol 12:539–547
He Y, Chung EH, Hubert DA, Tornero P, Dangl JL (2012) Specific missense alleles of the arabidopsis jasmonic acid co-receptor COI1 regulate innate immune receptor accumulation and function. PLos Genet 8:e1003018
Howe GA, Major IT, Koo AJ (2018) Modularity in jasmonate signaling for multistress resilience. Annu Rev Plant Biol 69:387–415
Huang H, Wang C, Tian H, Sun Y, Xie D, Song S (2014) Amino acid substitutions of GLY98, LEU245 and GLU543 in COI1 distinctively affect jasmonate-regulated male fertility in Arabidopsis. Sci China Life Sci 57:145–154
Huang H, Liu B, Liu L, Song S (2017) Jasmonate action in plant growth and development. J Exp Bot 68:1349–1359
Ishiguro S, Kawai-Oda A, Ueda J, Nishida I, Okada K (2001) The DEFECTIVE IN ANTHER DEHISCIENCE gene encodes a novel phospholipase A1 catalyzing the initial step of jasmonic acid biosynthesis, which synchronizes pollen maturation, anther dehiscence, and flower opening in Arabidopsis. Plant Cell 13:2191–2209
Katsir L, Schilmiller AL, Staswick PE, He SY, Howe GA (2008) COI1 is a critical component of a receptor for jasmonate and the bacterial virulence factor coronatine. Proc Natl Acad Sci USA 105:7100–7105
Kazan K (2015) Diverse roles of jasmonates and ethylene in abiotic stress tolerance. Trends Plant Sci 20:219–229
Kazan K, Manners JM (2008) Jasmonate signaling: toward an integrated view. Plant Physiol 146:1459–1468
Lee HY, Seo JS, Cho JH, Jung H, Kim JK, Lee JS, Rhee S, Choi YD (2013) Oryza sativa COI homologues restore jasmonate signal transduction in Arabidopsis coi1-1 mutants. PLoS ONE 8:e52802
Li L, Zhao Y, McCaig BC, Wingerd BA, Wang J, Whalon ME, Pichersky E, Howe GA (2004) The tomato homolog of CORONATINE-INSENSITIVE1 is required for the maternal control of seed maturation, jasmonate-signaled defense responses, and glandular trichome development. Plant Cell 16:126–143
Liao Y, Wei J, Xu Y, Zhang Z (2015) Cloning, expression and characterization of COI1 gene(AsCOI1) from Aquilaria sinensis (Lour.) Gilg. Acta Pharm Sin B 5:473–481
Maksymiec W, Wianowska D, Dawidowicz AL, Radkiewicz S, Mardarowicz M, Krupa Z (2005) The level of jasmonic acid in Arabidopsis thaliana and Phaseolus coccineus plants under heavy metal stress. J Plant Physiol 162:1338–1346
Mandaokar A, Thines B, Shin B, Lange BM, Choi G, Koo YJ, Yoo YJ, Choi YD, Choi G, Browse J (2006) Transcriptional regulators of stamen development in Arabidopsis identified by transcriptional profiling. Plant J 46:984–1008
Mann DG, Lafayette PR, Abercrombie LL, King ZR, Mazarei M, Halter MC, Poovaiah CR, Baxter H, Shen H, Dixon RA, Parrott WA, Neal Stewart C Jr (2012) Gateway-compatible vectors for high-throughput gene functional analysis in switchgrass (Panicum virgatum L.) and other monocot species. Plant Biotechnol J 10:226–236
Marone M, Mozzetti S, De Ritis D, Pierelli L, Scambia G. Semiquantitative (2001) RT-PCR analysis to assess the expression levels of multiple transcripts from the same sample. Biol Proced Online 3:19–25
McConn M, Browse J (1996) The critical requirement for linolenic acid is pollen development, not photosynthesis, in an Arabidopsis mutant. Plant Cell 8:403–416
McConn M, Creelman RA, Bell E, Mullet JE, Browse J (1997) Jasmonate is essential for insect defense in Arabidopsis. Proc Natl Acad Sci USA 94:5473–5477
Park JH, Halitschke R, Kim HB, Baldwin IT, Feldmann KA, Feyereisen R (2002) A knock-out mutation in allene oxide synthase results in male sterility and defective wound signal transduction in Arabidopsis due to a block in jasmonic acid biosynthesis. Plant J 31:1–12
Paschold A, Halitschke R, Baldwin IT (2007) Co(i)-ordinating defenses: NaCOI1 mediates herbivore-induced resistance in Nicotiana attenuata and reveals the role of herbivore movement in avoiding defenses. Plant J 51:79–91
Pauwels L, Inzé D, Goossens A (2009) Jasmonate-inducible gene: what does it mean? Trends Plant Sci 14:87–91
Pauwels L, Barbero GF, Geerinck J, Tilleman S, Grunewald W, Pérez AC, Chico JM, Bossche RV, Sewell J, Gil E, García-Casado G, Witters E, Inzé D, Long JA, De Jaeger G, Solano R, Goossens A (2010) NINJA connects the co-repressor TOPLESS to jasmonate signalling. Nature 464:788–791
Peng SQ, Xu J, Li HL, Tian WM (2009) Cloning and molecular characterization of HbCOI1 from Hevea brasiliensis. Biosci Biotechnol Biochem 73:665–670
Per TS, Khan MIR, Anjum NA, Masood A, Hussain SJ, Khan NA (2018) Jasmonates in plants under abiotic stresses: crosstalk with other phytohormones matters. Environ Exp Bot 145:104–120
Reymond P, Weber H, Damond M, Farmer EE (2000) Differential gene expression in response to mechanical wounding and insect feeding in Arabidopsis. Plant Cell 12:707–720
Riemann M, Haga K, Shimizu T, Okada K, Ando S, Mochizuki S, Nishizawa Y, Yamanouchi U, Nick P, Yano M, Minami E, Takano M, Yamane H, Iino M (2013) Identification of rice Allene Oxide Cyclase mutants and the function of jasmonate for defence against Magnaporthe oryzae. Plant J 74:226–238
Rowe HC, Walley JW, Corwin J, Chan EK, Dehesh K, Kliebenstein DJ (2010) Deficiencies in jasmonate-mediated plant defense reveal quantitative variation in Botrytis cinerea pathogenesis. PLoS Pathog 6:e1000861
Sanders PM1, Lee PY, Biesgen C, Boone JD, Beals TP, Weiler EW, Goldberg RB (2000) The Arabidopsis delayed dehiscence1 gene encodes an enzyme in the jasmonic acid synthesis pathway. Plant Cell 2:1041–1061
Sasaki-Sekimoto Y, Taki N, Obayashi T, Aono M, Matsumoto F, Sakurai N, Suzuki H, Hirai MY, Noji M, Saito K, Masuda T (2005) Coordinated activation of metabolic pathways for antioxidants and defence compounds by jasmonates and their roles in stress tolerance in Arabidopsis. Plant J 44:653–668
Schaller F, Schaller A, Stintzi A (2004) Biosynthesis and metabolism of jasmonates. J Plant Growth Regul 23:179–199
Schilmiller AL, Koo AJ, Howe GA (2007) Functional diversification of acyl-coenzyme A oxidases in jasmonic acid biosynthesis and action. Plant Physiol 143:812–824
Sheard LB, Tan X, Mao H, Withers J, Ben-Nissan G, Hinds TR, Kobayashi Y, Hsu FF, Sharon M, Browse J, He SY, Rizo J, Howe GA, Zheng N (2010) Jasmonate perception by inositol-phosphate-potentiated COI1-JAZ co-receptor. Nature 468:400–405
Shoji T, Ogawa T, Hashimoto T (2008) Jasmonate-induced nicotine formation in tobacco is mediated by tobacco COI1 and JAZ genes. Plant Cell Physiol 49:1003–1012
Staswick PE, Yuen GY, Lehman CC (1998) Jasmonate signaling mutants of Arabidopsis are susceptible to the soil fungus Pythium irregulare. Plant J 15:747–754
Stintzi A, Browse J (2000) The Arabidopsis male-sterile mutant, opr3, lacks the 12-oxophytodienoic acid reductase required for jasmonate synthesis. Proc Natl Acad Sci USA 97:10625–10630
Stintzi A, Weber H, Reymond P, Browse J, Farmer EE (2001) Plant defense in the absence of jasmonic acid: the role of cyclopentenones. Proc Natl Acad Sci USA 98:12837–12842
Thaler JS, Stout MJ, Karban R, Duffey SS (1996) Exogenous jasmonates simulate insect wounding in tomato plants (Lycopersicon esculentum) in the laboratory and field. J Chem Ecol 22:1767–1781
Thatcher LF, Manners JM, Kazan K (2009) Fusarium oxysporum hijacks COI1-mediated jasmonate signaling to promote disease development in Arabidopsis. Plant J 58:927–939
Thines B, Katsir L, Melotto M, Niu Y, Mandaokar A, Liu G, Nomura K, Sheng Y, Gregg AH, Browse J (2007) JAZ repressor proteins are targets of SCFCOI1 complex during jasmonate signaling. Nature 448:661–665
Thomma BP, Eggermont K, Penninckx IA, Mauch-Mani B, Vogelsang R, Cammue BP, Broekaert WF (1998) Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc Natl Acad Sci USA 95:15107–15111
Vijayan P, Shockey J, Lévesque CA, Cook RJ, Browse J (1998) A role for jasmonate in pathogen defense of Arabidopsis. Proc Natl Acad Sci USA 95:7209–7214
von Malek B, van der Graaff E, Schneitz K, Keller B (2002) The Arabidopsis male-sterile mutant dde2-2 is defective in the ALLENE OXIDE SYNTHASE gene encoding one of the key enzymes of the jasmonic acid biosynthesis pathway. Planta 216:187–192
Wang Z, Dai L, Jiang Z, Peng W, Zhang L, Wang G, Xie D (2005) GmCOI1, a soybean F-box protein gene, shows ability to mediate jasmonate-regulated plant defense and fertility in Arabidopsis. Mol Plant Microbe Interact 18:1285–1295
Wang W, Liu G, Niu H, Timko MP, Zhang H (2014) The F-box protein COI1 functions upstream of MYB305 to regulate primary carbohydrate metabolism in tobacco (Nicotiana tabacum L. cv. TN90). J Exp Bot 65:2147–2160
Wang W, Yang X, Ding Y, Yin G, Ma H, Zhang J, Shi X, Zhang D, Li J, Zhang H (2015) Functional analysis of COI1 genes in oilseed rape (Brassica napus L.). Sci Agric Sin 48:1882–1891
Wasternack C (2014) Action of jasmonates in plant stress responses and development—applied aspects. Biotechnol Adv 32:31–39
Wasternack C, Feussner I (2018) The oxylipin pathways: biochemistry and function. Annu Rev Plant Biol 69:363–386
Wasternack C, Hause B (2013) Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann Bot 111:1021–1058
Wasternack C, Strnad M (2016) Jasmonate signaling in plant stress responses and development—active and inactive compounds. N Biotechnol 33:604–613
Xiao Y, Chen Y, Charnikhova T, Mulder PP, Heijmans J, Hoogenboom A, Agalou A, Michel C, Morel JB, Dreni L, Kater MM, Bouwmeester H, Wang M, Zhu Z, Ouwerkerk PB (2014) OsJAR1 is required for JA-regulated floret opening and anther dehiscence in rice. Plant Mol Biol 86:19–33
Xie DX, Feys BF, James S, Nieto-Rostro M, Turner JG (1998) COI1: an Arabidopsis gene required for jasmonate-regulated defense and fertility. Science 280:1091–1094
Xu L, Liu F, Wang Z, Peng W, Huang R, Huang D, Xie D (2001) An Arabidopsis mutant cex1 exhibits constant accumulation of jasmonate-regulated AtVSP, Thi2.1 and PDF1.2. FEBS Lett 494:161–164
Yan C, Xie D (2015) Jasmonate in plant defence: sentinel or double agent? Plant Biotechnol J 13:1233–1240
Yan Y, Stolz S, Chételat A, Reymond P, Pagni M, Dubugnon L, Farmer EE (2007) A downstream mediator in the growth repression limb of the jasmonate pathway. Plant Cell 19:2470–2483
Yan J, Zhang C, Gu M, Bai Z, Zhang W, Qi T, Cheng Z, Peng W, Luo H, Nan F, Wang Z, Xie D (2009) The Arabidopsis CORONATINE INSENSITIVE1 protein is a jasmonate receptor. Plant Cell 21:2220–2236
Yan Y, Christensen S, Isakeit T, Engelberth J, Meeley R, Hayward A, Emery RJ, Kolomiets MV (2012) Disruption of OPR7 and OPR8 reveals the versatile functions of jasmonic acid in maize development and defense. Plant Cell 24:1420–1436
Yan Y, Borrego E, Kolomiets MV (2013) Jasmonate biosynthesis, perception and function in plant development and stress responses. In: Rodrigo VB (ed) Lipid metabolism, Chap. 16. InTech, Arlington, pp 393–442
Yang DL, Yao J, Mei CS, Tong XH, Zeng LJ, Li Q, Xiao LT, Sun TP, Li J, Deng XW, Lee CM, Thomashow MF, Yang Y, He Z, He SY (2012) Plant hormone jasmonate prioritizes defense over growth by interfering with gibberellin signaling cascade. Proc Natl Acad Sci USA 109:E1192–E1200
Zhai Q, Yan C, Li L, Xie D, Li C (2017) Jasmonates. In: Li J, Li C, Steven M, Smith SM (eds) Hormone metabolism and signaling in plants. Academic, New York, pp 243–272
Zhang G, Liu X, Quan Z, Cheng S et al (2012) Genome sequence of foxtail millet (Setaria italica) provides insights into grass evolution and biofuel potential. Nat Biotechnol 30:549–554
Zhang L, Zhang F, Melotto M, Yao J, He SY (2017) Jasmonate signaling and manipulation by pathogens and insects. J Exp Bot 68:1371–1385
Zhao Y, Dong W, Zhang N, Ai X, Wang M, Huang Z, Xiao L, Xia G (2014) A wheat allene oxide cyclase gene enhances salinity tolerance via jasmonate signaling. Plant Physiol 164:1068–1076
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31571580), the Fundamental Research Funds for the Central Universities (KYTZ201402 and KYRC201404), the Outstanding Scientific Innovation Team Program for Jiangsu Universities (2015), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). We are grateful to Dr. Chunhao Jiang (Nanjing Agricultural University, Nanjing, China) who provided the culture of Bortytis cinerea and to Dr. Daoxin Xie (Tsinghua University, Beijing, China) who provided the coi1-1 heterozygous Arabidopsis for the experiments of this study. We greatly thank Dr. Muhammad Aslam, University of Agriculture Faisalabad, Pakistan, for proof-reading the manuscript.
Author information
Authors and Affiliations
Contributions
The work presented here was carried out in collaboration among all the authors. LA performed the experiments, analyzed data and contributed to write the manuscript. YY designed the project, analyzed data, wrote the manuscript and obtained funds to support the project. RMA contributed to the experiment performance and the manuscript writing. HR and JQ helped to perform the experiments. All authors have read and approved the final manuscript.
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
An, L., Ahmad, R.M., Ren, H. et al. Jasmonate Signal Receptor Gene Family ZmCOIs Restore Male Fertility and Defense Response of Arabidopsis mutant coi1-1. J Plant Growth Regul 38, 479–493 (2019). https://doi.org/10.1007/s00344-018-9863-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-018-9863-2