Abstract
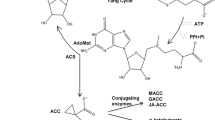
The dual effects of auxin and ethylene on rice seminal root growth were investigated in this study. Low concentrations of exogenous indole-3-acetic acid (IAA) had no effect on rice seminal root growth, whereas higher concentrations (≥0.003 μM) were inhibitory. In contrast, low concentrations of the auxin action inhibitor p-chlorophenoxyisobutyric acid (PCIB), ranging from 0.5 to 50 μM, promoted rice seminal root growth, whereas high concentrations of PCIB (≥500 μM) and the polar auxin transport inhibitor 2,3,5-triiodobenzoic acid (TIBA) inhibited rice seminal root growth. These results suggest that endogenous auxin is required but supraoptimal for rapid growth of rice seminal roots. In addition, although rice seminal root growth was inhibited by the exogenous ethylene-releasing compound ethephon or the ethylene precursor 1-aminocyclopropane-1-carboxylic acid (ACC) as well as exogenous IAA, the 50% inhibition of growth (I50) caused by ethephon or ACC was weakened by certain concentrations of the ethylene action inhibitor Ag+ (0.016-0.4 μM). However, the I50 caused by exogenous IAA was strengthened by Ag+ or the ethylene biosynthetic inhibitor aminoethoxyvinylglycine (AVG) and weakened by certain concentrations of PCIB (0.5-50 μM). Together, the inhibitory mechanisms of auxin and ethylene on rice seminal root growth should be different, and auxin inhibition of rice seminal root growth should not be caused by ethylene. Furthermore, our results indicated that a certain threshold level of ethylene was required to maintain rice seminal root growth, and that ethylene within the threshold may antagonize auxin inhibition of rice seminal root growth.


Similar content being viewed by others
References
Abel S, Nguyen MD, Chow W, Theologis A (1995) ASC4, a primary indoleacetic acid-responsive gene encoding 1-aminocyclopropane-1-carboxylate synthase in Arabidopsis thaliana. J Biol Chem 270:19093–19099
Abeles FB (1966) Auxin stimulation of ethylene evolution. Plant Physiol 41:585–588
Adams DO, Yang SF (1979) Ethylene biosynthesis: identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc Natl Acad Sci USA 76:170–174
Biswas KK, Ooura C, Higuchi K, Miyazaki Y, Nguyen VV, Rahman A, Uchimiya H, Kiyosue T, Koshiba T, Tanaka A, Narumi I, Oono Y (2007) Genetic characterization of mutants resistant to the antiauxin p-chlorophenoxyisobutyric acid reveals that AAR3, a gene encoding a DCN1-like protein, regulates responses to the synthetic auxin 2,4-dichlorophenoxyacetic acid in Arabidopsis roots. Plant Physiol 145:773–785
Bleecker AB, Estelle MA, Somerville C, Kende H (1988) Insensitivity to ethylene conferred by a dominant mutation in Arabidopsis thaliana. Science 241:1086–1089
Boller T, Herner R, Kende H (1979) Assay for and enzymatic formation of an ethylene precursor, 1-aminocyclopropane-1-carboxylic acid. Planta 145:293–303
Bucher D, Pilet PE (1983) Auxin effects on root growth and ethylene production. Experientia 39:493–494
Caderas D, Muster M, Vogler H, Mandel T, Rose JKC, McQueen-Mason S, Kuhlemeier C (2000) Limited correlation between expansin gene expression and elongation growth rate. Plant Physiol 123:1399–1413
Chadwick AV, Burg SP (1967) An explanation of the inhibition of root growth caused by indole-3-acetic acid. Plant Physiol 42:415–420
Chadwick AV, Burg SP (1970) Regulation of root growth by auxin-ethylene interaction. Plant Physiol 45:192–200
Chapman EJ, Estelle M (2009) Cytokinin and auxin intersection in root meristems. Genome Biol 10:210
Chhun T, Taketa S, Tsurumi S, Ichii M (2003) Interaction between two auxin-resistant mutants and their effects on lateral root formation in rice (Oryza sativa L.). J Exp Bot 54:2701–2708
Dolan L (1997) The role of ethylene in the development of plant form. J Exp Bot 48:201–210
Eliasson L, Bertell G, Bolander E (1989) Inhibitory action of auxin on root elongation not mediated by ethylene. Plant Physiol 91:310–314
Elmo M, Beyer JR (1976) A potent inhibitor of ethylene action in plants. Plant Physiol 58:268–271
Ferreira P, Hemerly A, Engler JDA, Bergounioux C, Burssens S, Montagu MV, Engler G, Inzé D (1994a) Three discrete classes of Arabidopsis cyclins are expressed during different intervals of the cell cycle. Proc Natl Acad Sci USA 91(24):11313–11317
Ferreira PC, Hemerly AS, Engler JD, Montagu MV, Engler G, Inzé D (1994b) Developmental expression of the Arabidopsis cyclin gene cyc1At. Plant Cell 6:1763–1774
Fu X, Harberd NP (2003) Auxin promotes Arabidopsis root growth by modulating gibberellin response. Nature 421:740–743
Guo J, Song J, Wang F (2007) Genome-wide identification and expression analysis of rice cell cycle genes. Plant Mol Biol 64:349–360
Guzmán P, Ecker JR (1990) Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell 2:513–523
Hahn A, Zimmermann R, Wanke D, Harter K, Edelmann HG (2008) The root cap determines ethylene-dependent growth and development in maize roots. Mol Plant 1:359–367
Heyn AN (1981) Molecular basis of auxin-regulated extension growth and role of dextranase. Proc Natl Acad Sci USA 78:6608–6612
Itoh H, Ueguchi-Tanaka M, Sato Y, Ashikari M, Matsuoka M (2002) The gibberellin signaling pathway is regulated by the appearance and disappearance of SLENDER RICE1 in nuclei. Plant Cell 14:57–70
Karahara I, Matsuda K, Honma Y (2008) Effects of ethylene on the production, elongation, and differentiation of endodermal cells in maize primary root: an integrative analysis of the developmental process of a particular cell type. Plant Root 2:29–37
Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR (1993) CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the Raf family of protein kinases. Cell 72:427–441
Kutschera U, Niklas KJ (2007) The epidermal-growth-control theory of stem elongation: an old and a new perspective. J Plant Physiol 164:1395–1409
Le J, Vandenbussche F, Van Der Straeten D, Verbelen JP (2004) Position and cell type-dependent microtubule reorientation characterizes the early response of the Arabidopsis root epidermis to ethylene. Physiol Plant 121:513–519
Li X, Mo X, Shou H, Wu P (2006) Cytokinin-mediated cell cycling arrest of pericycle founder cells in lateral root initiation of Arabidopsis. Plant Cell Physiol 47:1112–1123
Lorbiecke R, Sauter M (1999) Adventitious root growth and cell-cycle induction in deepwater rice. Plant Physiol 119:21–29
Ma Z, Baskin TI, Brown KM, Lynch JP (2003) Regulation of root elongation under phosphorus stress involves changes in ethylene responsiveness. Plant Physiol 131:1381–1390
Magyar Z, Veylder LD, Atanassova A, Bakó L, Inzé D, Bögre L (2005) The role of the Arabidopsis E2FB transcription factor in regulating auxin-dependent cell division. Plant Cell 17:2527–2541
McKay MJ, Ross JJ, Lawrence NL, Cramp RE, Beveridge CA, Reid JB (1994) Control of internode length in Pisum sativum. Plant Physiol 106:1521–1526
Métraux JP, Kende H (1983) The role of ethylene in the growth response of submerged deep water rice. Plant Physiol 72:441–446
Morgan PW, Hall WC (1962) Effect of 2,4-dichlorophenoxyacetic acid on the production of ethylene by cotton and grain sorghum. Physiol Plant 15:420–427
Mulkey TJ, Kuzmanoff KM, Evans ML (1982a) Promotion of growth and hydrogen ion efflux by auxin in roots of maize pretreated with ethylene biosynthesis inhibitors. Plant Physiol 70:186–188
Mulkey TJ, Kuzmanoff KM, Evans ML (1982b) Promotion of growth and shift in the auxin dose/response relationship in maize roots treated with the ethylene biosynthesis inhibitors aminoethoxyvinylglycine and cobalt. Plant Sci Lett 25:43–48
Oono Y, Ooura C, Rahman A, Aspuria ET, Hayashi K, Tanaka A, Uchimiya H (2003) p-Chlorophenoxyisobutyric acid impairs auxin response in Arabidopsis root. Plant Physiol 133:1135–1147
Pierik R, Tholen D, Poorter H, Visser EJ, Voesenek LA (2006) The Janus face of ethylene: growth inhibition and stimulation. Trends Plant Sci 11:176–183
Rahman A, Bannigan A, Sulaman W, Pechter P, Blancaflor EB, Baskin TI (2007) Auxin, actin and growth of the Arabidopsis thaliana primary root. Plant J 50(3):514–528
Richard C, Lescot M, Inzé D, Veylder LD (2002) Effect of auxin, cytokinin, and sucrose on cell cycle gene expression in Arabidopsis thaliana cell suspension cultures. Plant Cell Tissue Organ Cult 69:167–176
Richards DE, King KE, Ait-ali T, Harberd NP (2001) How gibberellin regulates plant growth and development: a molecular genetic analysis of gibberellin signaling. Annu Rev Plant Physiol Plant Mol Biol 52:67–88
Rodríguez FI, Esch JJ, Hall AE, Binder BM, Schaller GE, Bleecker AB (1999) A copper cofactor for the ethylene receptor ETR1 from Arabidopsis. Science 283:996–998
Roman G, Lubarsky B, Kieber JJ, Rothenberg M, Ecker JR (1995) Genetic analysis of ethylene signal transduction in Arabidopsis thaliana: five novel mutant loci integrated into a stress response pathway. Genetics 139:1393–1409
Růžička K, Ljung K, Vanneste S, Podhorská R, Beeckman T, Friml J, Benková (2007) Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell 19:2197–2212
Sakamoto T, Miura K, Itoh H, Tatsumi T, Ueguchi-Tanaka M, Ishiyama K, Kobayashi M, Agrawal GK, Takeda S, Abe K, Miyao A, Hirochika H, Kitano H, Ashikari M, Matsuoka M (2004) An overview of gibberellin metabolism enzyme genes and their related mutants in rice. Plant Physiol 134:1642–1653
Soeno K, Goda H, Ishii T, Ogura T, Tachikawa T, Sasaki E, Yoshida S, Fujioka S, Asami T, Shimada Y (2010) Auxin biosynthesis inhibitors, identified by a genomics-based approach, provide insights into auxin biosynthesis. Plant Cell Physiol 51(4):524–536
Soni R, Carmichael JP, Shah ZH, Murray JAH (1995) A family of cyclin D homologs from plants differentially controlled by growth regulators and containing the conserved retinoblastoma protein interaction motif. Plant Cell 7:85–103
Steen DA, Chadwick AV (1973) Effects of cycloheximide on indoleacetic acid-induced ethylene production in pea root tips. Plant Physiol 52:171–173
Stepanova AN, Hoyt JM, Hamilton AA, Alonso JM (2005) A link between ethylene and auxin uncovered by the characterization of two root-specific ethylene-insensitive mutants in Arabidopsis. Plant Cell 17:2230–2242
Stepanova AN, Yun J, Likhacheva AV, Alonso JM (2007) Multilevel interactions between ethylene and auxin in Arabidopsis roots. Plant Cell 19:2169–2185
Strader LC, Beisner ER, Bartel B (2009) Silver ions increase auxin efflux independently of effects on ethylene response. Plant Cell 21:3585–3590
Swarup R, Perry P, Hagenbeek D, Straeten DV, Beemster GT, Sandberg G, Bhalerao R, Ljung K, Bennett MJ (2007) Ethylene upregulates auxin biosynthesis in Arabidopsis seedlings to enhance inhibition of root cell elongation. Plant Cell 19:2186–2196
Tsuchisaka A, Theologis A (2004) Unique and overlapping expression patterns among the Arabidopsis 1-amino-cyclopropane-1-carboxylate synthase gene family members. Plant Physiol 136:2982–3000
Vanneste S, Maes L, Smet ID, Himanen K, Naudts M, Inzé D, Beeckman T (2005) Auxin regulation of cell cycle and its role during lateral root initiation. Physiol Plant 123:139–146
Wang KL-C, Li H, Ecker JR (2002) Ethylene biosynthesis and signaling networks. Plant Cell 14:S131–S151
Werner T, Motyka V, Laucou V, Smets R, Van Onckelen H, Schmüelling T (2003) Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell 15:2532–2550
Yamamoto Y, Kamiya N, Morinaka Y, Matsuoka M, Sazuka T (2007) Auxin biosynthesis by the YUCCA genes in rice. Plant Physiol 143:1362–1371
Yang SF, Hoffman NE (1984) Ethylene biosynthesis and its regulation in higher plants. Annu Rev Plant Physiol 35:155–189
Yin C, Gan L, Ng D, Zhou X, Xia K (2007) Decreased panicle-derived indole-3-acetic acid reduces gibberellin A1 level in the uppermost internode, causing panicle enclosure in male sterile rice Zhenshan 97A. J Exp Bot 58:2441–2449
Yoo S-D, Cho Y, Sheen J (2009) Emerging connections in the ethylene signaling network. Trends Plant Sci 14(5):270–279
Zarembinski TI, Theologis A (1993) Anaerobiosis and plant growth hormones induce two genes encoding 1-aminocyclopropane-1-carboxylate synthase in rice (Oryza sativa L.). Mol Biol Cell 4:363–373
Zhang N, Hasenstein KH (2002) 4,4,4-trifluoro-3-(indole-3-)-butyric acid promotes root elongation in Lactuca sativa independent of ethylene synthesis and pH. Physiol Plant 116:383–388
Zhao Y, Hasenstein KH (2009) Primary root growth regulation: the role of auxin and ethylene antagonists. J Plant Growth Regul 28:309–320
Zhou D-X, Yin K, Xu Z-H, Xue H-W (2003) Effects of polar auxin transport on rice root development. Acta Bot Sin 45(12):1421–1427
Zimmerman PW, Wilcoxon F (1935) Several chemical growth substances which cause initiation of roots and other responses in plants. Contributons Boyce Thompson Inst 7:209–229
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 30800669) and the New Scholar Foundation of the Doctoral Program of Ministry of Education of China (No. 200805041015).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yin, C., Wu, Q., Zeng, H. et al. Endogenous Auxin is Required but Supraoptimal for Rapid Growth of Rice (Oryza sativa L.) Seminal Roots, and Auxin Inhibition of Rice Seminal Root Growth is Not Caused by Ethylene. J Plant Growth Regul 30, 20–29 (2011). https://doi.org/10.1007/s00344-010-9162-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-010-9162-z