Abstract
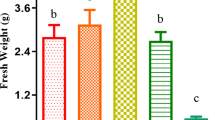
The present study was undertaken to test the influence of exogenously applied jasmonic acid (JA) at concentrations of 0.01–100 μM upon the growth and metabolism of the aquatic plant Wolffia arrhiza (Lemnaceae). JA acted in a concentration-dependent manner. JA at 0.1 μM stimulated plant growth and accumulation of cellular components (proteins, monosaccharides, chlorophylls, phaeophytins, and carotenoids). Treatment with JA at 0.1 μM enhanced W. arrhiza viability by the induction of biomass production and increased the level of photosynthetic pigments, monosaccharides, and soluble proteins. Moreover, JA at 0.1 μM activated the enzymatic (catalase, ascorbate peroxidase, NADH peroxidase) and nonenzymatic antioxidant (ascorbate, glutathione) system in W. arrhiza and, therefore, suppressed lipid peroxidation. In contrast, decreases in fresh weight, major photosynthetic pigments, monosaccharides, and soluble protein content were observed in W. arrhiza exposed to 100 μM JA. JA applied at 100 μM also stimulated the formation of lipid peroxides which are responsible for membrane damage. In the presence of 100 μM JA, antioxidant enzyme (catalase, ascorbate peroxidase, NADH peroxidase) activity and ascorbate as well as glutathione content were inhibited. The data support the hypothesis that JA plays an important role in W. arrhiza growth and metabolism, regulating oxidative status by direct influence on the enzymatic as well as nonenzymatic antioxidant machinery.





Similar content being viewed by others
References
Aeby H (1984) Catalase in vitro. Methods Enzymol 105:125–212
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress and signal transduction. Annu Rev Plant Biol 55:373–399
Appenroth KJ, Dathe W, Hertel W, Augsten H (1991) Photophysiology of turion germination in Spirodela polyrhiza (L.) SCHLEIDEN. VII. Action of jasmonic acid. J Plant Physiol 138:345–349
Bajguz A, Asami T (2005) Suppression of Wolffia arrhiza growth by brassinazole, an inhibitor of brassinosteroid biosynthesis and its restoration by endogenous 24-epibrassinolide. Phytochemistry 66:1787–1796
Bogatek R, Côme D, Corbineau F, Ranjan R, Lewak S (2002) Jasmonic acid affects dormancy and sugar catabolism in germinating apple embryos. Plant Physiol Biochem 40:167–173
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Carpin S, Crèvecoeur M, de Meyer M, Simon P, Greppin H, Claude P (2001) Identification of a Ca2+-pectate binding site on an apoplastic peroxidase. Plant Cell 13:511–520
Curtin C, Zhang W, Franco C (2003) Manipulating anthocyanin composition in Vitis vinifera suspension cultures by elicitation with jasmonic acid and light irradiation. Biotechnol Lett 25:1131–1135
Czerpak R, Piotrowska A, Szulecka K (2006) Jasmonic acid affects changes in the growth and some components content in alga Chlorella vulgaris. Acta Physiol Plant 28:195–203
Danon A, Miersch O, Felix G, op den Camp RGL, Apel K (2005) Concurrent activation of cell death-regulating signaling pathways by singlet oxygen in Arabidopsis thaliana. Plant J 41:68–80
De Kok LJ, Maas FM, Godeke J, Haaksma AB, Kuiper PJC (1986) Glutathione, a tripeptide which may function as a temporary storage compound of excessive reduced sulphur in H2S fumigated spinach plants. Plant Soil 91:349–352
Fujita M, Mori K, Kodera T (1999) Nutrient removal and starch production through cultivation of Wolffia arrhiza. J Biosci Bioeng 87:194–198
Gazaryan IG, Lagrimini LM, Ashby GA, Thorneley RNF (1996) The mechanism of indole-3-acetic acid oxidation by plant peroxidases: Anaerobic stopped-flow spectrophotometric studies on horseradish and tobacco peroxidases. Biochem J 313:841–847
Godziemba-Czyż J (1970) Characteristics of vegetative and resting forms of Wolffia arrhiza (L.) Wimm. II. Anatomy, physical and physiological properties. Acta Soc Bot Pol 39:421–443
He Y, Fukushige H, Hildebrand DF, Gan S (2002) Evidence supporting a role of jasmonic acid in Arabidopsis leaf senescence. Plant Physiol 128:876–884
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hutner SH (1953) Comparative physiology of heterotrophic growth in plants. In: Loomis WE (ed) Growth and differentiation in plants. Iowa State College Press, Ames, IA, pp 417–446
Ishida A, Ookubu K, Ono K (1987) Formation of hydrogen peroxide by NAD(P)H oxidation with isolated cell wall-associated peroxidase from cultured liverwort cells, Marchantia polymorpha L. Plant Cell Physiol 28:723–726
Kampfenkel K, Van Montagu M, Inzé D (1995) Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal Biochem 225:165–167
Krajnčič B, Nemec J (1995) The effect of jasmonic acid on flowering in Spirodela polyrrhiza (L.) Schleiden. J Plant Physiol 146:754–756
Krajnčič B, Kristl J, Janžekovič I (2006) Possible role of jasmonic acid in the regulation of floral induction, evocation and floral differentiation in Lemna minor L. Plant Physiol Biochem 44:752–758
Kristl J, Veber M, Krajnčič B, Oresnik K, Slekovec M (2005) Determination of jasmonic acid in Lemna minor (L.) by liquid chromatography with fluorescence detection. Anal Bioanal Chem 383:886–893
Kwak JM, Nguyen V, Schroeder JI (2006) The role of reactive oxygen species in hormonal response. Plant Physiol 141:323–329
Landolt E (1986) The family of Lemnaceae—a monographic study. Veröffentlichungen des Geobot Instituts ETH, vol 71. Stiftung Rübel, Zürich, pp 1–563
Landolt E, Kandeler R (1987) Biosystematic investigations in the family of duckweeds (Lemnaceae), Vol. 2, Phytochemistry, physiology, application, bibliography. Veröffentlichungen des Geobot Instituts ETH. Stiftung Rübel, Zürich, 638 pp
Les DH, Landolt E, Crawford DJ (1997) Systematics of Lemnaceae (duckweeds): inferences from micromolecular and morphological data. Plant Syst Evol 204:161–177
Maksymiec W, Krupa Z (2002) The in vivo and in vitro influence of methyl jasmonate on oxidative processes in Arabidopsis thaliana leaves. Acta Physiol Plant 24:351–357
Mical AH, Krotke A (1999) Wolffia arrhiza (L.)—small but strong. Acta Hydrobiol 41:165–170
Miyamoto K, Oka M, Ueda J (1997) Update in the possible mode of action of jasmonates: Focus on the metabolism of cell wall polysaccharides in relation to growth and development. Physiol Plant 100:631–638
Nakano Y, Asada K (1981) Hydrogen peroxidase is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Orozco-Cárdenas ML, Narváez-Vázquez J, Ryan CA (2001) Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wouding, systemin, and methyl jasmonate. Plant Cell 8:2309–2323
Pasternak TP, Prinsen E, Ayaydin F, Miskolczi P, Potters G, Asard H, van Onckelen HA, Dudits D, Feher A (2002) The role of auxin, pH, and stress in the activation of embryogenic cell division in leaf protoplast-derived cells of Alfalfa. Plant Physiol 129:1807–1819
Ranjan R, Lewak S (1995) Interaction of jasmonic acid in the control of lipases and proteases in germinating apple embryos. Physiol Plant 83:421–426
Reinbothe S, Reinbothe C, Heintzen C, Seidenbecher C, Parthier B (1993) A methyl jasmonate-induced shift in the length of the 5′ untranslated region impairs translation of the plastid rbcL transcript in barley. EMBO J 12:1505–1512
Saniewski M, Miszczak A, Kawa-Miszczak L, Węgrzynowicz-Lesiak E, Miyamoto K, Ueda J (1998) Effects of methyl jasmonate on anthocyanin accumulation, ethylene production, and CO2 evolution in uncooled and cooled tulip bulbs. J Plant Growth Regul 17:33–37
Somogyi M (1954) Notes on sugar determination. J Biol Chem 195:19–23
Świątek A, van Dongen W, Esmans EL, van Onckelen HA (2004) Metabolic fate of jasmonates in tobacco Bright Yellow-2 cells. Plant Physiol 135:161–172
Van Breusegen F, Dat JF (2006) Reactive oxygen species in plant cell death. Plant Physiol 141:384–390
Vèronèsi C, Pouénta ML, Riskauer M, Esquerré J, Tugay MT (1999) Regulation of tobacco lipoxygenase by methyl jasmonate and fatty acids. Plant Biol Pathol 322:491–497
Wasternack C (2007) Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann Bot 100:681–697
Wellburn AR (1994) The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144:307–313
Xiang C, Oliver DJ (1998) Glutathione metabolic genes coordinately respond to heavy metals and jasmonic acid in Arabidopsis. Plant Cell 10:1530–1550
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Piotrowska, A., Bajguz, A., Czerpak, R. et al. Changes in the Growth, Chemical Composition, and Antioxidant Activity in the Aquatic Plant Wolffia arrhiza (L.) Wimm. (Lemnaceae) Exposed to Jasmonic Acid. J Plant Growth Regul 29, 53–62 (2010). https://doi.org/10.1007/s00344-009-9113-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-009-9113-8