Abstract
A one-dimensional premixed ethylene–air flame is investigated regarding the presence of various combustion intermediates and products relevant for the formation of carbonaceous particles for various equivalence ratios and spatial positions using in situ UV–Vis absorption spectroscopy. A laser driven light source in combination with a fast spectrometer allow to record absorption spectra at a high rate required for practical combustion devices. The approach is coupled with a least squares regression procedure using a database of several absorbing species in the flame. To account for the high temperature flame conditions, the absorption spectra are convoluted by a simplified Maxwell–Boltzmann distribution model. While the approach is based on several assumptions and a verification requires future detailed intercomparison with other techniques, a first semi-quantitative evaluation can be obtained. This novel approach opens a potential route to the in situ measurement of the evolution of polycyclic aromatic hydrocarbons (PAHs) in flames.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Despite increasing electrification, combustion devices such as internal combustion engines will still play an important role for many years, especially in large vehicles like trucks or ships. For almost all practical combustion devices the reduction of soot emission, known as a hazardous pollutant, is of major importance. Besides the importance in emission reduction, particle formation from hydrocarbons is of high relevance, e.g., during jet fuel preheating in air-force systems [1] or the observation of interstellar matter [2,3,4]. Understanding the entire process chain of soot formation allows to control and influence certain formation steps and finally to avoid soot formation and thus emission.
During the soot formation process in fuel-rich combustion a gas to solid-phase transition occurs starting with the decomposition of the fuel/oxidizer mixture into radicals. In a next step, polycyclic aromatic hydrocarbons (PAHs) play a key role as soot precursors [5], from which three-dimensional structures start to form [6]. These grow further and built the first small particles known as incipient soot particles or soot nuclei consisting of several hundreds of atoms [7]. The terminology of these first particles during the transition from gas to solid-phase differs and was recently clarified by Michelsen et al. [8]. These particles were found to be transparent to visible light and were therefore often described as “transparent particles” or nano-organic carbon (NOC) [9,10,11,12]. The entire process is dependent on the fuel composition [13] and various other physical parameters, e.g., the flow field or ambient conditions such as temperature or pressure. Anyhow, the difficulty of gaining deeper knowledge about the formation process derives from the various species that are or can be involved. This is especially crucial in the modelling of soot formation, in which various reaction steps have to be considered precisely [14]. Measurement techniques capable of measuring the numerous different species and their concentrations are thus required.
For the identification of molecules usually sampling based ex situ techniques are applied such as gas chromatography and mass spectrometry (GC/MS) [15,16,17]. Although, these are reliable and quantitative tools to characterise different species, there are various drawbacks such as the disturbance of the flame through the probing device, the limited amount of sampled material, the limited sensitivity for larger molecules, and the potentially ongoing reaction and condensation of gas-phase species during the sampling procedure [18]. Similar limitations caused by the sampling procedure and ex situ measurement hinder other techniques such as atomic force microscopy (AFM) [19], jet-cooled laser-induced fluorescence (LIF) [20, 21] or molecular beam methods coupled with mass spectrometry (MBMS) [22], e.g., electron-impact ionization mass spectrometry [23]. To overcome these disadvantages optical methods play an important role in diagnostics [24], as they allow for fast in situ measurements with high spatial and/or temporal resolution and without disturbing the combustion process. For in situ PAH detection mainly fluorescence based techniques are used [25, 26], either applied as species selective technique or as a qualitative but 2D method. A fact that often hampers in situ PAH measurements is the formation and presence of solid soot particles besides the molecules leading to strong background signals.
Soot diagnostics can be subdivided into in situ and ex situ techniques as well. For latter, the use of ultrafine scanning mobility particle sizers (SMPS) [27, 28] or microscopy based techniques as helium-ion microscopy (HIM) [28, 29] or transmission electron microscopy (TEM) allow to draw a detailed picture of the soot particle size distribution during the formation process [30]. An overview of several in situ techniques to characterise the soot formation process was presented by Michelsen et al. [31]. While laser-induced incandescence (LII) allows to determine the concentration [32] and size of soot particles down to 1–2 nm [7, 28] elastic light scattering (ELS) techniques are capable of determining the morphology of soot aggregates. Minutolo et al. reported a combination of several optical techniques to characterise small transparent nanoparticles with a size of around 2–3 nm [9, 11, 12, 33] existing besides soot particles with approximately 10–50 nm in a laboratory flame. Their measurements included ultraviolet–visible (UV–Vis) absorption-, fluorescence- and scattering-techniques.
While first UV–Vis absorption spectroscopy was used for the examination of the soot formation process in a rather qualitative manner, D’Alessio et al. [9] used the Tauc equation to analyse the measured absorption spectra according to the optical band gap [34]. Miller et al. [35, 36] presented several studies on how this optical bandgap yields information about PAHs size according to the number of aromatic rings in a non-premixed ethylene/air nitrogen flame. A recent study revealed good agreements between in situ and ex situ extinction measurements according to optical band gap and the dispersion coefficient [37]. Further, ex situ species selective measurements were presented by Tregrossi et al. [38] based on a regression method on liquid samples taken from rich ethylene oxygen flames. Although such techniques based on a deconvolution of the signal require a database of the absorption spectra of each individual species present in the flame they yet allow for a simultaneous measurement of the species involved.
In this work, UV–Vis absorption spectroscopy is applied as an in situ line of sight technique in a 1-dimensional premixed C2H4 flat flame burner to investigate the early steps of soot formation. A fast spectrometer with a framerate of 4.5 kHz in combination with a broadband laser driven light source with a high spectral output power allow to acquire absorption spectra with down to 10 µs exposure time. The technique is coupled with a least squares regression procedure based on an absorption spectra database of the individual absorbing species, considering the high temperature ambient conditions by a simple Maxwell–Boltzmann-Distribution model. The main advantage of the technique presented is the species selective simultaneous determination of PAHs and soot. This allows to follow the production, growth and consumption of certain PAHs and soot formation at spatially resolved positions in the investigated flame.
2 Materials and methods
2.1 Experimental setup
For the broadband light emission necessary for UV–Vis absorption spectroscopy a laser driven light source (LDLS) (Energetiq, EQ-99XFC) is used similar to that in the work of Bouvier et al. [39]. In the LDLS, a continuous wave (CW) laser with a wavelength of 974 nm is focussed in a lamp housing to create a local plasma. Several optical elements in the housing image the broadband plasma into a solarization resistant multi-mode fibre with a core diameter of 230 µm. At the output, a beam with a numerical aperture of 0.22 is obtained. Compared to other UV light sources, e.g., a deuterium lamp, the spectrum is continuously distributed with a high spectral power over the spectral range from 190 to 2100 nm (> 10 µW/nm between 190 and 980 nm), where the intensity distribution over wavelength shows an increase from UV towards the visible region [40].
Due to the broadband light emission the use of lens fibre collimators (available with short focal lengths of a few mm) would lead to strong chromatic aberrations. Therefore, a parabolic aluminium mirror with a focal length of 20 mm is used to collimate the beam after the fibre output. With this collimator and the assumption of an ideal point light source a theoretically minimum beam diameter of approx. 9 mm can be achieved due to the numerical aperture. Measurements with a beam profiling camera (Ophir-Spiricon SP620U) show an almost top hat shaped beam profile with a minimum diameter of approx. 11 mm depicted in Fig. 1.
To ensure uniform spectral characteristics along the y-axis important for the measurements envisaged, we investigated the spatio-spectral properties of the beam. To this end, a glass fibre with a core diameter of 400 µm was put into the collimated UV–Vis beam to sample the beam spatially resolved, the spectrum was then measured using a customized spectrometer (Ocean Optics, Ocean FX) with a slit size of 50 µm and a spectral range from 200 to 540 nm. The results along the horizontal y-axis (black dashed line in Fig. 1A) are shown in Fig. 1B (absolute) and C (normalized to the maximum). As one can see, the spectrum is homogeneous across the beam’s y-direction and does not show significant spectral variations along this measurement line. Due to their low absolute intensity the spectral deviations at the outer beam boundaries (Fig. 1C) do not contribute significantly to the overall signal. This minimizes errors arising from beam steering.
A laminar premixed flat flame burner (McKenna type) with a stainless-steel sinter matrix and a stabilization plate 26 mm above the burner outlet is mounted on a translational stage to adjust the height for the measurements carried out (from 2 to 10 mm) covering the soot inception region. The overall gas flow is set constant at a flowrate of 10 l/min at laboratory conditions (20 °C, 1.0 bar), while the fuel- and air-flowrates are varied to the adjusted equivalence ratio. As fuel C2H4 was used. A nitrogen shroud gas flow at a flowrate of 15 l/min shields the flame from ambient air. We assume the flame to be 1-dimensional, i.e. the species distributions does only change with varying height above the burner surface (HAB), not with radial extent [41]. Moreover, strong effects from beam steering are minimized as the collimated light beam passes through the centre of the flame, which is 5–6 cm in diameter dependent on the HAB.
After transmission through the flame the collimated circular light beam (Fig. 1A) is focussed in the y-direction (horizontally) to increase the irradiance on the fibre input and thus on the spectrometer’s entrance slit. To this end, a cylindrical concave UV-enhanced aluminium mirror with a focal length of 50 mm is used, resulting in a vertically oriented beam profile as schematically depicted in the inset of Fig. 2B with a thickness of 0.8 mm and a height of 11 mm. The same fibre as described above is placed in the horizontal centre of this beam and the fibre’s diameter determines the vertical resolution of the measurement. The fibre is additionally mounted on a translational stage so it could be moved along z-direction. As the UV light emission is low compared to the VIS region, one aim of the experimental setup is to keep the amount of optical elements to a minimum, as each element especially reduces the spectral intensity at wavelengths < 230 nm. With the current setup, there is sufficient light to record spectra with an acquisition time down to 10 µs at a rate of 4.5 kHz.
A Experimental setup: The laser driven light source (a) with collimating mirror (b), the McKenna flat flame burner (c), the focussing cylindrical mirror (d) and fibre input (e) for the spectrometer (f). B Schematic top-view of the beam path with a cut of the z-y-plane and the fibre input position as inset
The measurement of I0 (as the incident light intensity) and I (light intensity after passing the absorbing media) are performed successively. As the intensity of the CW-laser driving the LDLS is modulated by approx. 50 MHz to avoid mode hopping an averaging over 1000 spectra is required. However, small remaining fluctuations in the spectra cannot be excluded completely, especially for low signal levels. As these fluctuations mainly affect all wavelengths of the spectrum in a similar manner and not only certain spectral bands, they result only in a small offset in the absorption spectra, which can easily be corrected during the evaluation.
2.2 Theoretical background
The measurement principle of absorption spectroscopy is based on Beer-Lambert’s law. In the following, a brief summary relevant for the evaluation of the measured data is given, for further information the reader is referred to the literature [42,43,44]. Under the assumption of a homogeneous and uniform species distribution along the line of sight of the measurement, the transmittance T of N species i at a certain wavelength λ is
Here, I0(λ) is the incident spectral intensity of the beam, I (λ) is the spectral intensity of the beam measured after passing the absorbing media of path length L. ni is the number density, σi(λ) the attenuation cross section and αi(λ) the absorption coefficient of species i. As the actually measured extinction is the sum of absorption and scattering the latter one has to be quantified or taken into account computationally. However, several studies showed that for low sooting flames and small aggregates scattering from soot particle aggregates is in the range of a few percent compared to absorption [45,46,47]. Therefore, we simplify that extinction is only due to absorption in this work. It should be reiterated that we focus on low HAB in this work, which aims at the investigation of the early stages of soot formation.
As mentioned in the introduction, different species are probably present at a certain position in the flame. Therefore, the light attenuation derives from contribution of the gas-phase species as well as the soot particles. While soot absorbs over a wavelength range from UV to IR [48], the aromatic compounds only show absorption peaks in the UV to VIS region. This derives from the required energy of a photon to excite an electron from the highest occupied molecular orbital (HOMO) to the lowest unoccupied molecular orbital (LUMO). This energy gap is associated with the bonding type in the molecule, which are mainly π–π* transitions for PAHs. Aliphatic compounds, which are known to exist in flames [49], mainly show σ–σ* transitions and therefore absorb at wavelengths below 200 nm, where also the oxygen and nitrogen in the ambient gas starts to absorb light. However, while species as C2H2 or CO2 do not show any absorption in the UV region above 200 nm at room temperature, at elevated temperatures (as obviously present in flames) their optical properties change. The influence of high temperature will be discussed in detail in an upcoming section.
2.3 The database
2.3.1 Selection of PAHs
As (to the best of the authors’ knowledge) there is no comprehensive database of absorption cross sections available for a large collection of PAHs, the database used is thus based on various references, e.g., the Spectral Atlas of Polycyclic Aromatic Compounds [50]. A major effort of the present work was the compilation of the species from the various reference sources and if necessary the conversion of their absorption characteristics into a common unit as listed in Table 1. It must be mentioned that the list of aromatic compounds is not complete and limited to small and medium sized PAHs with a maximum of 7 rings. This inherent limitation obviously affects the results and outcomes in the upcoming sections and mainly derives from the availability of absorption data. Yet the choice of species in this work is in large parts in consistency with those reported in recent publications by, e.g., Mercier et al. [25] or Zhang et al. [26]. Larger aromatic structures or dimers that are known to exist in the flame (as considered by Mercier et al. and Zhang et al.) are not accessible at this stage.
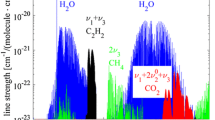
First the spectra from the various references were transferred to the same physical unit: the absorption cross section σ in cm2 (Fig. 3). In addition, an interpolation to 1 nm steps was performed as the spectral resolution differs for the various references and 1 nm seems to be an appropriate step width for a graphical evaluation if no explicit data points were given. The different diagrams are arranged according to the number of aromatic rings, starting from the gas-phase compounds and benzene (upper left diagram) up to seven ring PAHs and soot (lower right diagram). Obviously, the main absorption bands are shifted towards higher wavelengths with increasing size of the molecule or increasing number of aromatic rings, while pronounced absorption in the visible can only be observed for the soot spectrum. Although the absorption cross section of soot can be orders of magnitude higher compared to those of PAHs, it differs from those of the PAHs depicted in Fig. 3 by the absorption in the VIS region. Only soot shows a continuous absorption towards the IR wavelength region; PAHs still exhibit clear absorption bands.
Wavelength dependent absorption cross sections of individual compounds following Table 1 and the references therein. Each diagram shows spectra with increasing size of molecules or number of rings, starting from gaseous compounds and one ring aromatics (upper left) to > 6 ring aromatics and soot (lower right). For presentation, the soot absorption cross section is multiplied by a factor of 10–4 in case of the assumption of the measured soot spectrum taken from the literature (\({d}_{\mathrm{p}}=\) 20 nm) and a factor of 10–2 for the calculated soot spectrum (\({d}_{\mathrm{p}}=\) 5 nm), based on a constant E(m(λ)). Scaling differences in soot spectra arise from different assumptions of \({d}_{\mathrm{p}}\)
2.3.2 Contribution of soot
In this work, we used a soot absorption spectrum based on calculations using the Rayleigh approximation,
where E(m(λ)) is the absorption function with the wavelength dependent complex index of refraction m. As there are hardly reported values for E(m) in the deep UV region [65] we assume it to be constant with E(m) = 0.2 [66] in this work and used the assumption of \({d}_{\mathrm{p}}=\) 5 nm [67] (referring to soot actually present at the measurement positions in the flame) for the computation of \({\sigma }_{\mathrm{soot}}\). As pointed out by Michelsen et al. [68] soot optical properties such as E(m(λ)) very much depend on soot maturity and thus chemical composition [32, 66, 69]. Moreover, the absorption properties also depend on the internal atomic structure of soot primary particles as highlighted by Fernandez et al. [70]. We want to emphasize that the assumption of a constant E(m) used in this work is just one possible choice among many others with different variations over wavelength, yet still resulting in a continuous absorption spectrum.
To reveal the influence of the chosen soot absorption spectrum, we additionally used a soot absorption spectrum (given in arb. units) that was measured by Tregrossi and Ciajolo [64] on soot sampled from a flame. We normalized the spectrum to its maximum value, multiplied it with the maximum value of the soot absorption coefficient presented by Sgro et al. [33] (in cm−1) and calculated the absorption cross section assuming a particle diameter \({d}_{\mathrm{p}}\) of 20 nm and a soot volume fraction \({f}_{V}\) of 0.4 ppm [71] (referring to mature soot for the collected samples). This particle size does not match with the first assumption of 5 nm. However, despite the fact that a difference in dp alters the soot absorption cross section by a constant factor (∝ dp3), the spectral behaviour is independent of dp. Different assumptions for \({d}_{\mathrm{p}}\) thus influence the resulting number densities of soot particles, and therefore no meaningful values for the soot number density can be directly obtained. Yet, the model described in “Evaluation method” requires all entries with the same physical unit σ ([σ] = cm2), so that number densities of soot are the quantities that are directly inferred. Using the obtained number densities and applying the same assumption for the particle size dp meaningful \({f}_{V}\) values could be computed (irrespective of the original assumptions). Both soot spectra (measured and calculated) are shown in red colour in the lower right subplot of Fig. 3. With the option chosen here and with the experimental spectrum from the literature we used two quite distinct absorption spectra (the first one with a monotonous decrease, the second one with a maximum at approx. 240 nm), which may exemplarily demonstrate the influence of the soot absorption spectrum on the inferred PAHs profiles, which is discussed at the end of “Results and discussion”. Other results shown are obtained with the calculated spectrum as standard setting. It should be underlined that for a reliable determination of soot concentrations, the choice of an appropriate absorption cross section is of paramount importance, and there is ample work in the literature on the soot absorption properties, see e.g. [69] and references therein. This is not in the focus of the present work, yet the consideration of soot as a major absorber deems necessary to judge about its potential influence on determination of other species, and an approximate measurement of soot concentration completes the picture regarding the evolution of the various PAHs.
Possible wavelength-independent intensity fluctuations of the light source could lead to a biased signal (constant over all wavelengths). To account for this effect a variable, but wavelength-independent offset (with a reference value arbitrarily chosen on the order of typical values for σ) is added as another contribution to the spectrum besides the 25 species in the database for the upcoming evaluation.
2.3.3 Temperature effects
Comparing the absorption spectra of the various compounds in Fig. 3 to measured ones (cf. Fig. 5) one can see that individual peaks of the database are smoothened in the measured data. This effect arises from the different ambient conditions regarding the measurements and simulations conducted in the literature to obtain the database spectra and those conducted in this work. Some species were measured in the liquid phase in the literature, which could lead to deviations compared to the gas-phase spectra measured in this work. Due to clustering- and solvent effects in liquids slight energetic influences among molecules lead to broader absorption bands. Besides, the pressure and, most importantly, the temperatures differ significantly. The optical properties change at elevated temperatures. As most of the spectra in the database were measured at ambient temperature (≈ 300 K), a model is required to account for the changed optical properties under flame temperatures (≈ 1750 K).
At flame temperatures, due to the higher thermal energy, more molecules occupy higher energetic levels than in the cold/ambient case. Therefore, a redshift (lower photon energy) in the absorption peaks can be observed (as less photon energy is required to excite the transitions). Moreover, the redshift is accompanied by a broadening and diminishing of the single peaks. The behaviour can be approximated by the Maxwell–Boltzmann-Distribution for the energy fE(E), simplified as [43]
Here, kB is Boltzmann’s constant, Eth = kBT is the thermal energy of the system depending on the temperature T and E is the energy of the molecule. With increasing temperature T, the mean and median energy as well as the width of the distribution increase. By accounting for the temperature effect first, the wavelengths of the absorption spectra are converted to energies following \(E_{{\upgamma }} = \left( {h \cdot c} \right)/\left( {\lambda \cdot e} \right)\) with c as the speed of light, e as elementary charge and h as Planck’s constant ([\({E}_{\upgamma }\)] = eV). Then each line of the spectra is convoluted with the Boltzmann kernel of Eq. (3) for an elevated temperature. Finally the broadened energy-absorbance-spectra are converted back to wavelength. Through this procedure a shift towards longer wavelength as well as a broadening of single peaks can be observed. As the spectra reported in the database were mainly measured or modelled at room temperature, an overestimation of the redshift due to the temperature difference of about 300 K between measurement and Eq. (3) must be avoided. This in principle would first require a deconvolution of the database spectra to obtain non-broadened and non-shifted spectra at low temperature (~ 0 K). However, as this deconvolution itself is prone to error in this work a simplification is made. To this end, the thermal energy shift at the corresponding database temperature \({T}_{0}\) was subtracted according to \({E}_{\mathrm{th},\mathrm{red}}={k}_{\mathrm{B}}T-{k}_{\mathrm{B}}{T}_{0}\) resulting in a reduced thermal energy with a nonlinear wavelength shift, which is increasing for increasing wavelengths.
To validate this simplified energetic model, measurements on 1-methyl-naphthalene were conducted in a continuously scavenged gas flow cell as described in previous work [72]. A comparison between modelled and measured absorption spectra is depicted in Fig. 4. The solid lines show measurements of 1-methyl-naphthalene spectra at three different temperatures (350 K, 550 K, 750 K). Applying the convolution kernel of Eq. (3) and the energy-offset correction for the temperatures of 550 K (green lines) and 750 K (red lines) to the measured spectrum at 350 K (blue line) one obtains the dashed lines depicted in Fig. 4. For T = 550 K the measurement results in the cell and the modelled data are in very good agreement. For T = 750 K data the deviations are more pronounced. However, the general trends of the redshift are quite well reproduced and allow for a more accurate evaluation of measurement data compared to the room-temperature spectra shown in Fig. 3. With this simplified model of Eq. (3) the entire database can be convoluted for high temperatures. As accurate temperatures were not available for all measurement positions and equivalence ratios under investigation in this work, and as for high temperatures of about 1750 K variations in T of about 200 K are of lower influence on the simulated absorption spectra, we assumed an average constant flame temperature of 1750 K.
2.4 Evaluation method
Following Eq. (1), the spectrally resolved measurement of I and I0 at K different wavelengths and the knowledge of the absorption length L at a certain HAB allows to calculate the absorption coefficient vector \({\boldsymbol{\alpha }}_{\mathbf{m}\mathbf{e}\mathbf{a}\mathbf{s}}={\left[\alpha ({\lambda }_{1}),\alpha ({\lambda }_{2}), \dots , \alpha ({\lambda }_{K})\right]}^{\uptau }\) (\(\uptau\) for transposed). The quantities of interest (QoI) are a vector \({{\varvec{n}}}_{\mathrm{QoI}}\) containing the number densities of each individual species with
With the database containing the absorption cross sections σi of each species i, it is possible to model an effective absorption coefficient vector:
To obtain the QoI a regression between measured and modelled data is performed. To this end, a least-square method is applied to minimize the residuals. Then
where j denotes the spectral measurement position \({\lambda }_{j}\). \({{\varvec{n}}}_{\mathrm{MLE}}\) is a vector containing the maximum likelihood estimates (MLE), i.e. the solution of the best fit, for the number density of each species.
3 Results and discussion
Figure 5 shows example spectra of the absorption coefficient for different equivalence ratios at a HAB of 7 mm for C2H4 flames calculated following Eq. (1).
In general, the absorption coefficient increases with increasing equivalence ratio for all wavelengths. As shown in Fig. 6, with the bare eye one can observe the first soot being formed as a slight orange glim in the flame between Φ = 1.9 and Φ = 2.1. For the measurements in flames with lower equivalent ratios almost no absorption is present in the visible region, yet a peak exists in the deep UV region (λ < 250 nm). The broad band absorption in the visible range due to absorption by soot particles increases with increasing Φ, at the same time the absorption peak is slightly redshifted.
With the regression method presented in the previous section “Evaluation method”, data for C2H4 flames at several HAB positons and equivalence ratios were evaluated. The wavelength for evaluation ranges from 215 to 539 nm in 1 nm steps. For smaller wavelengths the conversion of the database to flame temperature following the procedure described in “The database” leads to boundary effects. To avoid these artefacts the spectra between 200 and 215 nm are not evaluated. Figure 7 shows the measurements of the absorption coefficients in black and the model fit using the \({{\varvec{n}}}_{\mathrm{MLE}}\) values as input parameters for the simulation in colour. Obviously, the measurement and model are in good agreement. For the low sooting flames (Φ ≤ 1.9) or at low HAB in the flame (HAB < 5 mm) an offset of the spectra can be observed deriving mainly from the intensity fluctuations in the LDLS. These fluctuations are most pronounced for low attenuation through the medium, as then small variations in the overall intensity show the greatest effect on the determined absorption, yet it diminishes with increasing absorption. In the model we account for these fluctuations by a variable offset (constant over wavelength). The method is supposed to be capable of distinguishing between the broadband soot absorption and the constant offset as the soot spectrum shows a remaining slope in the visible region, decreasing towards larger wavelength.
Figure 8 depicts the number densities of all species for five different equivalence ratios and two heights above the burner surface, namely 2 and 7 mm HAB. The species in Fig. 8 are arranged so that the size of the molecules increases from left to right. The smallest molecule in the database (CO2) is on the left, the large solid soot particles are second last, while the constant offset, accounting for intensity fluctuations of the light source, is last on the right.
Most likely number densities (entries of nMLE) for each species from the database for five different Φ at 2 and 7 mm HAB. Note the species-specific scaling in the diagrams for better visibility. The species without physical meaning are labelled with a * symbol. Fluctuations in spectra arising from the LDLS are accounted for by a variable offset (constant over wavelength) depicted in absolute values
For 2 mm HAB position and Φ = 1.8 or Φ = 1.9 no soot is observable even at 7 mm HAB. Due to the assumptions made in the previous section to calculate the absorption cross section of soot the number densities show high uncertainty and should be treated with extreme caution. Yet by converting them into soot volume fractions (using the same assumptions) the resulting values are in the same order as those presented by Simonsson et al. [73] as shown in Fig. 9.
Regarding the different PAHs under investigation, an overall shift to larger PAHs can be observed comparing both of the HAB data as well as with increasing Φ. This is reasonable as the PAHs grow with residence time. Some of the more stable ones, especially pyrene, seem to enrich with increasing Φ as well as with residence time while small ones as naphthalene seem to be consumed. Generally, the number density of naphthalene as a small aromatic species is expected to be higher than this of larger PAHs. Therefore, in this work its overall number density seems to be too low, which indicates problems and large uncertainties with the measurement of this species, which are probably due to the fact that naphthalene exhibits strong absorption especially in the range about 210 nm (at room temperature), i.e. in a range that is not completely accessible here. Beside pyrene, there are further prominent PAHs such as benzo[a]pyrene, benzo[b]fluoranthene or acenaphthylene. The main focus of the approach lies in the analysis of the PAHs contribution to the absorption measurements, while the absorption characteristics of species as NO2, CO2 or C2H2 interfere with those of PAHs at elevated temperatures and are therefore contained in the database. Yet, it should be mentioned that their determination is not the main purpose and that they rather act as nuisance parameters similar to the offset.
For a more detailed discussion, concentration profiles over HAB are compared with complementary techniques for soot, two PAH classes and benzene as a gaseous species.
As shown in Fig. 9, the soot volume fraction increases with increasing Φ as well as with an increase in HAB. For Φ = 2.5 there is a strong increase from 5 mm on, which trend is in general agreement with soot profiles reported for a slightly different Φ = 2.5 flame by thermocouple particle densitometry (different flow rates and dilution gases) [49]. Similar accordance can be observed with LII measurements taken in Φ = 2.1 and Φ = 2.3 flames from Simonsson et al. [73]. However, as stated, soot volume fractions obtained in this work are of semi-quantitative nature and were not the main focus of the present approach. The general trends are in good agreement with the results from the literature for similar flames (difference regarding the height of the stabilization plate; here 26 mm HAB; Simonsson et al. 21 mm HAB) (Fig. 10).
A qualitative comparison of C16H10 (pyrene and fluoranthene) as well as C24H10 profiles as exemplary PAH species is in compliance with GC/MS measurements from Ciajolo et al. [15] for two flames similar to those for Φ = 2.0 and Φ = 2.3 in this work. In the present work the flowrates are slightly higher leading to a small shift of the concentration peak positions to higher HABs compared to the results obtained by Ciajolo et al. [15]. Yet, for both PAH classes on average the profiles are in good agreement. However, some artefacts, e.g., in form of increased values of n at the 2–3 mm positions of C16H10 remain in the present evaluation.
The benzene profile shown in Fig. 11 reveals a concentration peak at 4 mm, which is in agreement with previous work in a similar flame [15]. Obviously, there are also some remaining artefacts resulting, e.g., in low concentrations at 5 mm and 6 mm HAB and also for the soot profile of Fig. 9 at 4 mm for the flame with Φ = 2.5. Similar artefacts can be observed in the bar-charts of Fig. 8, where, e.g., perylene is present at one certain measurement condition and position, which might not be a reasonable observation. These artefacts derive from the deconvolution process, which (at this point) lacks any regularization as a possibility to consider additional information of the inverse problem and is therefore likely to create some outliers as mentioned earlier for the concentration of naphthalene molecules.
To evaluate the stability of the approach Fig. 12 reveals how the temperature and the amount of species used in the database influence the resulting concentrations. To that end, the data presented in Fig. 8 for Φ = 1.9 at 7 mm HAB (green) are deconvoluted with a database temperature of 1550 K (blue) and 1950 K (red). Further, a deconvolution with the correct database temperature but an exclusion of the NO2 spectrum is presented in grey. It can be seen that the overall concentration profiles are not drastically altered for the changed database conditions. Some slight variations can be observed but no shift towards different species distributions occurs. Therefore, we assume that the approach is stable and leads to reliable estimates.
Most likely number densities (entries of nMLE) for each species from the database for different database temperatures and database entries at 7 mm HAB for Φ = 1.9 flame. The green bars correspond to the data presented in Fig .8. Blue and red bars correspond to a database temperature of 1550 and 1950 K, respectively. In grey the measured spectra were deconvoluted with a database (1750 K) without the NO2 spectrum
To investigate the influence of the soot absorption spectrum used in the database, the data of the flame with Φ = 2.1 were evaluated using the calculated and the measured soot spectra of Fig. 3 for both HAB positions of Fig. 8.
Figure 13 reveals that the inferred PAHs concentrations are hardly affected by the soot spectrum for both HABs. The approach for the measurement of PAHs is thus stable against changes of the soot absorption spectrum due to changing soot maturity. As mentioned above, a detailed intercomparison to other techniques could not be provided and therefore the presented approach should be seen as a first attempt of in situ species selective measurements and the results have to be treated with caution concerning their absolute values.

taken from measurements and once calculated as depicted in Fig. 3
Most likely number densities (entries of nMLE) for each species from the database for a Φ = 2.1 flame. Blue and purple bars are 2 mm HAB position while red and orange bars refer to 7 mm HAB. For both HAB positions the underlying soot absorption spectra was once
While a detailed uncertainty analysis using advanced statistical methods is beyond the scope of this work, we want to address some of the main limitations of the present approach. First, the species present in the database and the quality of the data with regard to the flame conditions is considered as the most important issue. Missing species that are actually present in the flame can only be compensated by other species with a similar absorption behaviour leading to their misinterpretation. This is complicated by the availability of data and the fact that the database is unlikely to be “complete”. Second, a more detailed validation of the herein suggested temperature model performed at flame temperatures for suitable species would be desirable. Therefore, our simplified energy-model may lead to additional uncertainties in the results. Moreover, a verification of the approach is required by a comparison with established techniques such as GC/MS. But generally, the comparison of in situ technique with methods requiring sampling techniques is all but straightforward, furthermore different sensitivities to the various species certainly pose a major problem. In essence, the approach presented is certainly associated with considerable uncertainty that cannot yet be quantified. In consequence the herein presented results should rather be interpreted in a semi-quantitative than an absolute way, allowing for an observation of trends regarding species evolution. While the method provides a promising approach for the in situ measurement of relevant species in fuel-rich flames and the investigation the early steps of soot formation, further work is required to improve the underlying databases and to validate uncertainties for the individual species.
4 Conclusion
In this work, an approach using in situ UV–Vis absorption spectroscopy for the determination of soot precursors in premixed fuel-rich C2H4 flames was presented. The technique allows to characterise several species simultaneously, based on a database and a least squares fitting procedure. To account for the high temperature ambient conditions a simplified Maxwell–Boltzmann-Distribution model was proposed. The results are in agreement with other measurement techniques shown for the examples: benzene as a gaseous species, pyrene + fluoranthene and coronene as prominent PAH molecules and soot as solid particle. Further, the influence of changed conditions of the database was investigated to check the stability of the approach. This relatively cheap, simple and fast technique may thus help to trace the soot formation process in plenty of different applications. In addition, it might help to gain a deeper understanding of the soot formation process and follow a stepwise growth from PAHs to soot particles.
Yet, the concept presented in this work suffers from the limitation of sequential recording and hence remaining intensity fluctuations of the light source. The use of two spectrometers and a simultaneous detection of I and I0 should solve this problem in future investigations. Further, a more detailed and appropriate database will help to improve the results. To that end, studies on pure PAHs at elevated temperatures are required. Also, while there is only minor influence on the determination of other species, the varying optical properties of soot during the growth processes should be considered for the determination of soot concentration. The major limitations so far, however, are the uncertainties in the underlying databases for the individual PAHs and the quantification of uncertainties in the determination of the individual species. The latter might be obtained in comparative investigations on the soot formation process in dedicated burners such as a Wolfhard-Parker or low pressure counterflow diffusion burners in future work.
References
E.A. Hurst, N.B. Poddar, K. Vutukuru, S.V. Kalpathy, M.J. Wornat, Polycyclic aromatic hydrocarbons formation and growth during the supercritical pyrolysis of 1-octene. Proc. Combust. Inst. 37(1), 1107–1115 (2019)
G. Malloci, G. Mulas, C. Joblin, Electronic absorption spectra of PAHs up to vacuum UV-towards a detailed model of interstellar PAH photophysics. Astron. Astrophys. 426(1), 105–117 (2004)
T. Henning, F. Salama, Carbon in the Universe. Science 282(5397), 2204–2210 (1998)
G. Sloan, E. Lagadec, A. Zijlstra, K. Kraemer, A. Weis, M. Matsuura, K. Volk, E. Peeters, W. Duley, J. Cami, Carbon-rich dust past the asymptotic giant branch: aliphatics, aromatics, and fullerenes in the magellanic clouds. Astrophys J 791(1), 28 (2014)
H. Wang, Formation of nascent soot and other condensed-phase materials in flames. Proc. Combust. Inst. 33(1), 41–67 (2011)
K. Johansson, M. Head-Gordon, P. Schrader, K. Wilson, H. Michelsen, Resonance-stabilized hydrocarbon-radical chain reactions may explain soot inception and growth. Science 361(6406), 997–1000 (2018)
H. Bladh, N.-E. Olofsson, T. Mouton, J. Simonsson, X. Mercier, A. Faccinetto, P.-E. Bengtsson, P. Desgroux, Probing the smallest soot particles in low-sooting premixed flames using laser-induced incandescence. Proc. Combust. Inst. 35(2), 1843–1850 (2015)
H.A. Michelsen, M.B. Colket, P.-E. Bengtsson, A. D’Anna, P. Desgroux, B.S. Haynes, J.H. Miller, G.J. Nathan, H. Pitsch, H. Wang, A review of terminology used to describe soot formation and evolution under combustion and pyrolytic conditions. ACS Nano 14(10), 12470–12490 (2020)
A. D’Alessio, A. D’Anna, G. Gambi, P. Minutolo, The spectroscopic characterisation of UV absorbing nanoparticles in fuel rich soot forming flames. J. Aerosol Sci. 29(4), 397–409 (1998)
A. D’Anna, A. Rolando, C. Allouis, P. Minutolo, A. D’Alessio, Nano-organic carbon and soot particle measurements in a laminar ethylene diffusion flame. Proc. Combust. Inst. 30(1), 1449–1456 (2005)
P. Minutolo, G. Gambi, A. D’Alessio, S. Carlucci, Spectroscopic characterisation of carbonaceous nanoparticles in premixed flames. Atmos. Environ. 33(17), 2725–2732 (1999)
P. Minutolo, G. Gambi, A. D’Alessio, Properties of carbonaceous nanoparticles in flat premixed C2H4/air flames with C/O ranging from 0.4 to soot appearance limit. Symposium (International) on Combustion, Elsevier 27(1), 1461–1469 (1998)
L. Ruwe, K. Moshammer, N. Hansen, K. Kohse-Höinghaus, Influences of the molecular fuel structure on combustion reactions towards soot precursors in selected alkane and alkene flames. Phys. Chem. Chem. Phys. 20(16), 10780–10795 (2018)
W. Pejpichestakul, E. Ranzi, M. Pelucchi, A. Frassoldati, A. Cuoci, A. Parente, T. Faravelli, Examination of a soot model in premixed laminar flames at fuel-rich conditions. Proc. Combust. Inst. 37(1), 1013–1021 (2019)
A. Ciajolo, A. D’Anna, R. Barbella, A. Tregrossi, The formation of aromatic carbon in sooting ethylene flames. Twenty-Fifth Symposium (International) on Combustion, Elsevier 25(1), 679–685 (1994)
K. Kohse-Höinghaus, A. Schocker, T. Kasper, M. Kamphus, A. Brockhinke, Combination of laser- and mass-spectroscopic techniques for the investigation of fuel-rich flames. Z. Phys. Chem. 219(5), 583 (2005)
C.S. McEnally, L.D. Pfefferle, B. Atakan, K. Kohse-Höinghaus, Studies of aromatic hydrocarbon formation mechanisms in flames: progress towards closing the fuel gap. Prog. Energy Combust. Sci. 32(3), 247–294 (2006)
P. Minutolo, A. D’Anna, A. D’Alessio, On detection of nanoparticles below the sooting threshold. Combust. Flame 152(1–2), 287–292 (2008)
F. Schulz, M. Commodo, K. Kaiser, G. De Falco, P. Minutolo, G. Meyer, D. Andrea, L. Gross, Insights into incipient soot formation by atomic force microscopy. Proc. Combust. Inst. 37(1), 885–892 (2019)
X. Mercier, M. Wartel, J.-F. Pauwels, P. Desgroux, Implementation of a new spectroscopic method to quantify aromatic species involved in the formation of soot particles in flames. Appl. Phys. B 91(2), 387–395 (2008)
M. Wartel, J.-F. Pauwels, P. Desgroux, X. Mercier, Pyrene measurements in sooting low pressure methane flames by jet-cooled laser-induced fluorescence. J. Phys. Chem. A 115(49), 14153–14162 (2011)
F. Defoeux, V. Dias, C. Renard, P. Van Tiggelen, J. Vandooren, Experimental investigation of the structure of a sooting premixed benzene/oxygen/argon flame burning at low pressure. Proc. Combust. Inst. 30(1), 1407–1415 (2005)
T.S. Kasper, P. Oßwald, M. Kamphus, K. Kohse-Höinghaus, Ethanol flame structure investigated by molecular beam mass spectrometry. Combust. Flame 150(3), 220–231 (2007)
P. Desgroux, X. Mercier, K.A. Thomson, Study of the formation of soot and its precursors in flames using optical diagnostics. Proc. Combust. Inst. 34(1), 1713–1738 (2013)
X. Mercier, O. Carrivain, C. Irimiea, A. Faccinetto, E. Therssen, Dimers of polycyclic aromatic hydrocarbons: the missing pieces in the soot formation process. Phys. Chem. Chem. Phys. 21(16), 8282–8294 (2019)
Y. Zhang, B. Xiao, Y. Li, P. Liu, R. Zhan, Z. Huang, H. Lin, LIF diagnostics for selective and quantitative measurement of PAHs in laminar premixed flames. Combust. Flame 222, 5–17 (2020)
A.D. Abid, J. Camacho, D.A. Sheen, H. Wang, Quantitative measurement of soot particle size distribution in premixed flames–the burner-stabilized stagnation flame approach. Combust. Flame 156(10), 1862–1870 (2009)
C. Betrancourt, F. Liu, P. Desgroux, X. Mercier, A. Faccinetto, M. Salamanca, L. Ruwe, K. Kohse-Höinghaus, D. Emmrich, A. Beyer, Investigation of the size of the incandescent incipient soot particles in premixed sooting and nucleation flames of n-butane using LII, HIM, and 1 nm-SMPS. Aerosol Sci. Technol. 51(8), 916–935 (2017)
M. Schenk, S. Lieb, H. Vieker, A. Beyer, A. Gölzhäuser, H. Wang, K. Kohse-Höinghaus, Imaging nanocarbon materials: soot particles in flames are not structurally homogeneous. ChemPhysChem 14(14), 3248–3254 (2013)
A. Baldelli, U. Trivanovic, T.A. Sipkens, S.N. Rogak, On determining soot maturity: A review of the role of microscopy-and spectroscopy-based techniques. Chemosphere 252, 126532 (2020)
H. Michelsen, Probing soot formation, chemical and physical evolution, and oxidation: a review of in situ diagnostic techniques and needs. Proc. Combust. Inst. 36(1), 717–735 (2017)
C. Betrancourt, X. Mercier, F. Liu, P. Desgroux, Quantitative measurement of volume fraction profiles of soot of different maturities in premixed flames by extinction-calibrated laser-induced incandescence. Appl. Phys. B 125(1), 16 (2019)
L. Sgro, G. Basile, A. Barone, A. D’Anna, P. Minutolo, A. Borghese, A. D’Alessio, Detection of combustion formed nanoparticles. Chemosphere 51(10), 1079–1090 (2003)
J. Tauc, R. Grigorovici, A. Vancu, Optical properties and electronic structure of amorphous germanium. phys. stat. sol. b 15(2), 627–637 (1966)
J.H. Miller, J.D. Herdman, C.D. Green, E.M. Webster, Experimental and computational determinations of optical band gaps for PAH and soot in a N2-diluted, ethylene/air non-premixed flame. Proc. Combust. Inst. 34(2), 3669–3675 (2013)
E.M. Adkins, J.H. Miller, Extinction measurements for optical band gap determination of soot in a series of nitrogen-diluted ethylene/air non-premixed flames. Phys. Chem. Chem. Phys. 17(4), 2686–2695 (2015)
C. Russo, B. Apicella, A. Tregrossi, A. Ciajolo, K.C. Le, S. Török, P.-E. Bengtsson, Optical band gap analysis of soot and organic carbon in premixed ethylene flames: comparison of in-situ and ex-situ absorption measurements. Carbon 158, 89–96 (2020)
A. Tregrossi, B. Apicella, A. Ciajolo, C. Russo, Fast analysis of PAH in complex organic carbon mixtures by reconstruction of UV-visible spectra. Chem. Eng. Trans. 57, 1447–1452 (2017)
M. Bouvier, J. Yon, G. Lefevre, F. Grisch, A novel approach for in-situ soot size distribution measurement based on spectrally resolved light scattering. J. Quant. Spectrosc. Radiat. Transfer 225, 58–68 (2019)
H. Zhu, P. Blackborow, Laser-Driven Light Sources for Nanometrology Applications. J. Microelec. Manufac. 2, 19020104 (2019)
F. Migliorini, S. De Iuliis, F. Cignoli, G. Zizak, How “'flat”’ is the rich premixed flame produced by your McKenna burner? Combust. Flame 153(3), 384–393 (2008)
K.C. Cossel, E.M. Waxman, I.A. Finneran, G.A. Blake, J. Ye, N.R. Newbury, Gas-phase broadband spectroscopy using active sources: progress, status, and applications. JOSA B 34(1), 104–129 (2017)
N.M. Laurendeau, Statistical Thermodynamics: Fundamentals and Applications (Cambridge University Press, 2005)
T.G. Mayerhöfer, J. Popp, Beer’s law derived from electromagnetic theory. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 215, 345–347 (2019)
B. Ma, M.B. Long, Combined soot optical characterization using 2-D multi-angle light scattering and spectrally resolved line-of-sight attenuation and its implication on soot color-ratio pyrometry. Appl. Phys. B 117(1), 287–303 (2014)
F. Liu, K. Thomson, G. Smallwood, Numerical investigation of the effect of signal trapping on soot measurements using LII in laminar coflow diffusion flames. Appl. Phys. B 96(4), 671–682 (2009)
C.M. Sorensen, Light scattering by fractal aggregates: a review. Aerosol Sci. Technol. 35, 648–687 (2001)
T.C. Bond, R.W. Bergstrom, Light absorption by carbonaceous particles: an investigative review. Aerosol Sci. Technol. 40(1), 27–67 (2006)
B. Öktem, M.P. Tolocka, B. Zhao, H. Wang, M.V. Johnston, Chemical species associated with the early stage of soot growth in a laminar premixed ethylene–oxygen–argon flame. Combust. Flame 142(4), 364–373 (2005)
W. Karcher, Spectral Atlas of Polycyclic Aromatic Compounds: Including Data on Physico-Chemical Properties, Occurrence and Biological Activity (Springer Science & Business Media, 2013)
C. Schulz, J. Koch, D. Davidson, J. Jeffries, R. Hanson, Ultraviolet absorption spectra of shock-heated carbon dioxide and water between 900 and 3050 K. Chem. Phys. Lett. 355(1–2), 82–88 (2002)
S. Zabeti, M. Fikri, C. Schulz, Ultraviolet absorption and laser-induced fluorescence of shock-heated acetylene. Proc. Combust. Inst. 36(3), 4469–4475 (2017)
H. Keller-Rudek, G.K. Moortgat, R. Sander, R. Sörensen, The MPI-Mainz UV/VIS spectral atlas of gaseous molecules of atmospheric interest. Earth System Science Data 5(2), 365–373 (2013)
A. Dawes, N. Pascual, S.V. Hoffmann, N.C. Jones, N.J. Mason, Vacuum ultraviolet photoabsorption spectroscopy of crystalline and amorphous benzene. Phys. Chem. Chem. Phys. 19(40), 27544–27555 (2017)
H. Grosch, Z. Sárossy, H. Egsgaard, A. Fateev, UV absorption cross-sections of phenol and naphthalene at temperatures up to 500° C. J. Quant. Spectrosc. Radiat. Transfer 156, 17–23 (2015)
P.J. Linstrom, W.G. Mallard, The NIST Chemistry WebBook: a chemical data resource on the internet. J. Chem. Eng. Data 46(5), 1059–1063 (2001)
S.K. Samanta, E. Preis, C.W. Lehmann, R. Goddard, S. Bag, P.K. Maiti, G. Brunklaus, U. Scherf, One-step synthesis of a cyclic 2, 17-dioxo [3, 3](4, 4′) biphenylophane and first preparation of a microporous polymer network from a macrocyclic precursor by cyclotrimerization. Chem. Commun. 51(43), 9046–9049 (2015)
H. Shemer, K.G. Linden, Aqueous photodegradation and toxicity of the polycyclic aromatic hydrocarbons fluorene, dibenzofuran, and dibenzothiophene. Water Res. 41(4), 853–861 (2007)
M. Taniguchi, J.S. Lindsey, Database of absorption and fluorescence spectra of> 300 common compounds for use in photochem CAD. Photochem. Photobiol. 94(2), 290–327 (2018)
M.M. Barichello, D. Bohrer, C. Viana, L.M. Carvalho, P.C. Nascimento, Determination of polycyclic aromatic hydrocarbons in commercial parenteral formulations and medications using high-performance liquid chromatography with diode array detection. J. AOAC Int. 100(4), 1070–1076 (2017)
J.W. Lu, J.M. Flores, A. Lavi, A. Abo-Riziq, Y. Rudich, Changes in the optical properties of benzo [a] pyrene-coated aerosols upon heterogeneous reactions with NO2 and NO3. Phys. Chem. Chem. Phys. 13(14), 6484–6492 (2011)
C. Wang, J. Wang, N. Wu, M. Xu, X. Yang, Y. Lu, L. Zang, Donor–acceptor single cocrystal of coronene and perylene diimide: molecular self-assembly and charge-transfer photoluminescence. RSC Adv. 7(4), 2382–2387 (2017)
J.W. Patterson, The ultraviolet absorption spectra of coronene. J. Am. Chem. Soc. 64(6), 1485–1486 (1942)
A. Tregrossi, A. Ciajolo, Spectral signatures of carbon particulate evolution in methane flames. Combust. Sci. Technol. 182(4–6), 683–691 (2010)
J. Yon, R. Lemaire, E. Therssen, P. Desgroux, A. Coppalle, K. Ren, Examination of wavelength dependent soot optical properties of diesel and diesel/rapeseed methyl ester mixture by extinction spectra analysis and LII measurements. Appl. Phys. B 104(2), 253–271 (2011)
N.-E. Olofsson, J. Simonsson, S. Török, H. Bladh, P.-E. Bengtsson, Evolution of properties for aging soot in premixed flat flames studied by laser-induced incandescence and elastic light scattering. Appl. Phys. B 119(4), 669–683 (2015)
S. De Iuliis, S. Maffi, F. Migliorini, F. Cignoli, G. Zizak, Effect of hydrogen addition on soot formation in an ethylene/air premixed flame. Appl. Phys. B 106(3), 707–715 (2012)
H.A. Michelsen, P.E. Schrader, F. Goulay, Wavelength and temperature dependences of the absorption and scattering cross sections of soot. Carbon 48(8), 2175–2191 (2010)
F. Liu, J. Yon, A. Fuentes, P. Lobo, G.J. Smallwood, J.C. Corbin, Review of recent literature on the light absorption properties of black carbon: refractive index, mass absorption cross section, and absorption function. Aerosol Sci. Technol. 54(1), 33–51 (2020)
C.G. Fernández, S. Picaud, M. Devel, Calculations of the mass absorption cross sections for carbonaceous nanoparticles modeling soot. J. Quant. Spectrosc. Radiat. Transfer 164, 69–81 (2015)
B. Axelsson, R. Collin, P.-E. Bengtsson, Laser-induced incandescence for soot particle size and volume fraction measurements using on-line extinction calibration. Appl. Phys. B 72(3), 367–372 (2001)
U. Retzer, H. Ulrich, F.J. Bauer, S. Will, L. Zigan, UV absorption cross sections of vaporized 1-methylnaphthalene at elevated temperatures. Appl. Phys. B 126(3), 1–6 (2020)
J. Simonsson, N.-E. Olofsson, S. Török, P.-E. Bengtsson, H. Bladh, Wavelength dependence of extinction in sooting flat premixed flames in the visible and near-infrared regimes. Appl. Phys. B 119(4), 657–667 (2015)
Acknowledgements
The LDLS has been provided by the Erlangen Graduate School in Advanced Optical Technologies (SAOT), and the authors gratefully acknowledge funding of the SAOT by the Bavarian State Ministry for Science and Art. We would like to thank Ulrich Retzer and Hannah Ulrich for their support during the measurements of absorption spectra of 1-methyl-naphthalene at elevated temperatures.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bauer, F.J., Degenkolb, M.U.J., Huber, F.J.T. et al. In situ characterisation of absorbing species in stationary premixed flat flames using UV–Vis absorption spectroscopy. Appl. Phys. B 127, 115 (2021). https://doi.org/10.1007/s00340-021-07664-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00340-021-07664-z