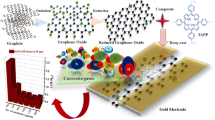
Abstract
The structural diversity and high surface reactivity of the metal–organic frameworks (MOFs) offer an ideal material platform for various applications such as gas storage, gas separation, catalyst, etc. However, their use in chemiresistive gas sensing is limited due to the requirement of optimized gas adsorption properties with electrical conductivity. In the present investigation, we have modulated the electrical properties of zinc benzene dicarboxylate (Zn-BDC) MOF by modifying it with partially reduced graphene oxide (rGO). The Zn-BDC and rGO composite (Zn-BDC@rGO) was synthesized by utilizing a solvothermal method and multiparametrically tested by various techniques such as X-Ray diffraction (XRD), UV–visible spectroscopy, Raman spectroscopy, Fourier transform infrared spectroscopy (FTIR), atomic force microscopy (AFM), thermogravimetric analysis (TGA), and I–V characteristics, for its structural, spectroscopic, morphological, surface area analysis, thermal stability, and electrical characterization, respectively. The synthesized Zn-BDC@rGO composite was deposited via drop casting method on the copper electrodes on a glass substrate (100 µm gap) using the shadow mask technique by the e-beam evaporator, and tested for the detection of ammonia, carbon monoxide, and sulfur dioxide using chemiresistive modality. The principal component analysis (PCA) indicates that the developed sensor selectively discriminates among the NH3, CO, and SO2 gases with low response/recovery time, i.e., 60/120 s at 20 ppm, which is far below the permissible exposure limit (PEL) suggested by The Occupational Safety and Health Administration (OSHA), USA for CO and NH3 and very close to the PEL level of SO2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Prosperity of modern society always relies on the industrial growth of any country. To a greater or lesser extent, this development needs the combustion of fossil fuels, which causes the emission of primary air pollutants, i.e., unburnt hydrocarbon, carbon monoxide, nitrogen oxide, etc. [1]. Moreover, interaction with sunlight and atmospheric oxygen leads to the generation of secondary pollutants like NO2, O3, NH4NO3, etc. [2]. Polluted air disturbs the ecosystem and causes many health issues, mainly in the cardiac, pulmonary, and vascular systems [3]. Therefore, in recent decades, the critical importance of detection of primary as well as secondary pollutants has regularly increased due to their toxicological effects on the environment and human health [4]. Globally, air pollution is linked to millions of deaths each year. According to WHO, nine out of ten people breathe polluted air worldwide, which may cause mortality risk [5]. In Asia, estimated deaths due to outdoor air pollution lead to 3.3 million per year, and attributed deaths in Canada are 21,000 per year [6]. The gradual decomposition of the NH4NO3 leads to the release of ammonia which is the most regnant alkaline gas in the atmosphere, and it plays a vital role in acid deposition and atmospheric chemistry, ammonia can transverse over long distances in the form of ammonium aerosols, thus contributing a pollutant on the international scale [7, 8]. Combustion of fossil fuels and the activity of the metallurgical and chemical plant releases million tons of SO2 and CO [9]. When SO2 gas combines with rain water, it forms sulfuric acid and leads to acid rain. Whereas, CO has long-term health effects on human body as well as can lead to many complications like headache, dizziness and drowsiness, nausea and vomiting, chest pain, loss of consciousness, pregnancy complications, and even death too [10]. Therefore, detection of NH3, SO2, and CO from the environment is necessary. Sensors having the capability of timely, accurate, and selective discrimination among the analytes need to be developed on priority.
In the past decade, materials like zeolite, conducting polymer, metal oxide, porphyrins, etc., have been explored in the field of chemical sensing. Still, graphene and its derivative have been found to be supreme in the field of chemical sensing because of their exciting properties like high carrier mobility, chemical stability, and electrical properties. Moreover, carbon-based composite materials like controlled functionalization of carbon nanotubes by poly N-methyl pyrrole [11], polyaniline/graphene oxide (GO) composite [12], graphitic nanoribbon films [13], GO hybrids [14], nanoparticle decorated graphene [15], etc. are examples of the materials which have shown great sensing properties because these materials show p-type behavior, when exposed to reducing gases and the positive charge carriers (hole), play a dominant role in charge transfer. Because of the depletion of the hole, materials resistivity increases [16, 17]. Nowadays, reduced graphene oxide (rGO) is widely used for sensing application due to its wide surface area, high defect sites, and high charge carrier mobility. It shows very effective sensing properties at room temperature [18]. Despite its high sensitivity, pristine rGO shows very high response and recovery time with poor selectivity toward reducing gases. Therefore, it must be functionalized to improve response recovery time and selectivity [19]. Whereas, an attractive class of symmetrical structured crystalline coordination polymers called metal–organic frameworks (MOFs) is formed by metal clusters and organic ligands, due to their exceptional structural characteristics, like tunable pore size, large surface area, catalytic activity, and large active sites [20]. Extensive work has been done on MOFs over the past decade. The MOFs signifies the new class of promising materials for chemical sensing because various charge transfer occurs between various ligands and metal centers [21]. To enhance sensitivity and selectivity, the specific qualities of MOF can be applied [22]. Up to date, many MOFs have been explored to detect air pollutants by various modalities, viz. luminescence [23], fluorescence [24], work function [25], quartz crystal microbalance(QCM) [26], etc. However, these modalities have limited their use because of their complex analysis techniques, low sensitivity, high power consumption, and high cost. On the other hand, chemiresistive sensor offers advantages like simple analysis, cost-effectiveness, and low power consumption. Due to such inherent simplicity, the chemiresistive modality attracts the attention of researchers worldwide. Many researchers have used MOF to detect various gas pollutants, such as Travlou et al., which have detected ammonia using Cu-based metal organic framework and graphene oxide hybrid [27]. Cho et al. integrated porous and conductive metal organic frameworks and detected H2S at room temperature [28]. Ko et al. used M3(HHTP)2/graphite to detect ammonia [29]. Michael G. Campbell et al. used HITP-Cu-HHTP for VOC detection [30]. Yao et al. used MOF thin film coated with a metal oxide nanowire array to improve VOC sensing [31]. Dmello et al. detected acidic gases using amine functionalized Zirconium MOF [32] Although all these sensors offer high sensitivity but it suffers from the high-temperature operation and very high response/recovery time with cross selectivity.
To cope up with the long-term bottleneck like selectivity and high response/recovery time, we have synthesized a composite of zinc benzenedicarboxylate framework and thermally reduced graphene oxide (rGO) to combine the uniqueness of graphene layers with MOFs. Although the composites Zn-BDC@GO have been reported previously, but it cannot be used for chemiresistive gas sensing since both the Zn-BDC and GO has low electrical conductivity; hence, we choose rGO instead of GO and first-time synthesized Zn-BDC@rGO composite and explored it in chemiresistive sensing modality [33, 34].
Zn-BDC is a primitive cubic structure in which benzene dicarboxylates (1,4-benzene dicarboxylate, BDC) form the junction between zinc oxide bands and [Zn4O]6þ tetrahedra inhabit the corners of the cubic crystal [34]. In this study, the composites of Zn-BDC with 20 wt% of rGO were synthesized and tested for chemiresistive gas sensing performance toward pollutants like ammonia (NH3), carbon monoxide (CO), and sulfur dioxide (SO2). Moreover, principal component analysis confirms that the fabricated sensor selectively discriminates among ammonia (NH3), carbon monoxide (CO), and sulfur dioxide (SO2), which would be useful for the development of the multianalyte sensing device.
2 Experimental
2.1 Materials and methods
Sulfuric acid (H2SO4), hydrochloric acid (HCl), graphite flakes, sodium nitrate, and hydrogen peroxide (H2O2) were obtained from Molychem, Mumbai, India. N, N-Dimethylformamide (DMF), 1,4-benzene dicarboxylate, zinc nitrate hexahydrate, and potassium permagnet were procured from Sigma-Aldrich, Darmstadt, Germany.
2.2 Synthesis of graphene oxide and reduced graphene oxide
GO has been oxidized from graphite using the hummers method [35]. Briefly, sodium nitrate (1 g) and graphite powder (0.6 g) were mixed slowly into H2SO4 (25 mL); after that, 3 g of KMnO4 was added slowly into the solution while stirring the resultant solution into an ice bath [maintaining temperature lower than (18 ºC)] for 2 h. Then, the ice bath was taken out, and the solution was stirred for 2 h. (30 ºC) until it became waxy brownish. Then, deionized (DI) water (100 ml) was added to the mixture. As a result of DI addition, hydration heat was increased with effervesces, and the combination changed the color to brown. Again, 200 mL of DI water was mixed into the reaction and stirred continuously. The reaction was terminated by treating the solution with 8 mL of H2O2. The mixture was purified by washing with 8% HCl and DI water 4–5 times with the help of centrifugation. Lastly, the mixture dried under vacuum at 60 ºC, and GO powder was collected.
The partial reduction of graphene oxide was done by thermal treatment. Briefly, 2 g of GO powder was treated at 200 ºC for 10 min to obtain partially reduced graphene oxide [36].
2.3 Preparation of Zn-BDC
The Zn-BDC MOF was prepared solvothermally, as reported elsewhere [37]. Briefly,70 ml N,N-dimethylformamide (DMF) was added in a mixture of zinc nitrate hexahydrate (5.2 g) and 1,4-benzene dicarboxylate (1 g) and stirred for 30 min at 550 rpm. The resultant solution is shifted into the Teflon-lined autoclave and kept in a preheated oven at 120 ºC for 25 h. After natural cooling, the crystals were cleaned with DMF and dipped in fresh chloroform overnight. Zn-BDC crystals were then collected and dried under a vacuum oven and activated at 130 °C for 24 h.
2.4 Synthesis of Zn-BDC@rGO composite
The Zn-BDC@rGO composite was prepared by the same solvothermal method mentioned above. Additionally, rGO suspension was mixed in the precursor solution. In detail, 70 ml N,N-dimethylformamide (DMF) was added in a mixture of zinc nitrate hexahydrate (5.2 g), 1,4-benzene dicarboxylate (1 g), and thermally reduced graphene oxide (20 wt%) and stirred for 30 min at 550 rpm. The resultant solution is shifted into the Teflon-lined autoclave and kept in a preheated oven at 120 ºC for 25 h. After cooling, the crystals were cleaned with DMF and dipped in fresh chloroform overnight. Zn-BDC@rGO composite crystals were then collected, dried, activated by as process as mentioned above, and stored for further use.
2.5 Characterizations
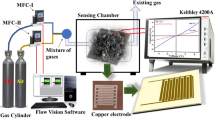
The X-ray diffraction pattern was obtained using an X-ray diffractometer (Bruker D8 Advanced, Rheinstetten, Karlsruhe, Germany) utilizing CuKα (λ = 1.54069 Å) radiation. Horiba Confocal Raman microscope (Xplora Plus, Horiba France SAS, Les Ulis Cedex, France) using 532 nm laser and 100 × objective with 1800 g/mm grating and Bruker Alpha FTIR (Rheinstetten, Karlsruhe, Germany) were used to study the materials chemical composition and molecular structure. The UV–visible spectroscopy of materials was studied on the UV–visible spectrophotometer (Jasco V-750, JASCO Corporation, Tokyo, Japan). The morphological studies were performed on an atomic force microscope in non-contact mode (Park XE-7, Park Systems Corporation, Seoul, S. Korea). The indigenously fabricated dynamic gas sensing setup and Keithley-4200A semiconductor parameter analyzer (Tektronix, Inc. Beaverton, Oregon, USA) were used to perform gas sensing experiments and current–voltage characteristics. Thermogravimetric analysis was carried out using the DTG-60H analyzer (Shimadzu Corporation, Kyoto, Japan) in the nitrogen environment. Principal component analysis was done by Origin 9 Pro software.
3 Results and discussion
3.1 X-ray diffraction
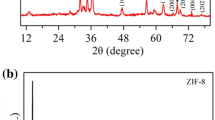
The XRD graph of GO, rGO, pristine Zn-BDC, and composite with thermally reduced GO, i.e., Zn-BDC@rGO, is represented in Fig. 1. The sharp peaks at 2θ = 11.09° and 45.02° validate the presence of (0 0 1) and (1 1 0) plane of graphene oxide, respectively. The decrease in the sharp diffraction peak at (11.08°) shows the elimination of some functional groups while converting GO into rGO [38]. The diffraction peak at 2θ = 24° in rGO shows the successful reduction of GO and formation of rGO. The XRD plot of the composite is matched with the pristine Zn-BDC; it agrees with those previously reported data [37]. The XRD pattern is preserved in our composites, which indicates that the appearance of rGO did not disturb the formation of bonding between Zn metal ion and ligand. The decrease in the intensity of peaks of composite materials shows a reduction in crystallinity because of the presence of rGO.
3.2 FTIR spectroscopy
The FTIR pattern of Zn-BDC, Zn-BDC@rGO, GO, and rGO are represented in Fig. 2, respectively. The intense peaks at 3400 cm−1 and 1720–1740 cm−1 of GO correspond to the O–H stretching of carboxylic groups and C=O stretching of carbonyl or carboxyl groups, respectively. The weak peak between 1590 and 1620 cm−1 is attributed to C=C skeletal vibration of the unoxidized graphic domain, and a broad peak at 1048 cm−1 is attributed to alkoxy C–O stretching vibration. The formation of graphene oxide from graphite flake can be confirmed by the appearance of all the above functional groups. After thermal treatment at 200 °C, the absence of peaks near 3400 cm−1 and 1048 cm−1 confirms the removal of O–H and alkoxy stretching vibration, which reveals the partial reduction of graphene oxide flakes[39].
The composite and parent materials major FTIR pattern matches with the reported data. In Zn-BDC, the peaks at 1510 cm−1, 1580 cm−1, and 1390 cm−1 confirm the presence of asymmetric and symmetric stretching of the carboxylic group, respectively. Peaks at 1300–700 cm−1 are because of out-of-plane vibration of the BDC linker [37].
3.3 UV–visible spectroscopy
The UV–visible spectra of GO and thermally reduced GO are depicted in Fig. 3a. The peak at 232 nm in GO spectra is caused by the π–π* transitions in the aromatic C–C bonds, and the weak hump at 300 nm is due to the presence of the n–π transition of C=O bonds. When treated thermally, the peak at 250 nm is attributed to the restoration of electronic conjugation within the graphene sheets[40]. Figure 3b shows the UV–visible spectra of Zn-BDC and its composite with thermally reduced graphene oxide. The strong absorption peak near 300 nm is the characteristic peak of Zn-BDC. The small peaks near 228 nm, 250 nm, and 262 nm indicate the presence of rGO in the Zn-BDC@rGO composite. The inset of Fig. 3b shows the tauc plot of Zn-BDC and Zn-BDC@rGO. The optical band gaps calculated from Tauc plot for Zn-BDC and Zn-BDC@rGO was 3.6 eV and 3.827 eV, respectively. The decrease in the band gap of Zn-BDC MOF after incorporation of rGO confirms that, electron exchange between Zn-BDC and rGO occurred in the composite and forms the heterojunction.
3.4 Raman spectroscopy
GO, rGO, Zn-BDC, and Zn-BDC@rGO are studied through Raman spectroscopy, and the results are shown in Fig. 4a, b. The Raman spectra of graphene oxide exhibit the D band at 1350 cm−1 due to 2 g mode first-order scattering. Whereas the G band at 1598 cm−1 is attributed to the degree of disorder. When GO is treated thermally at 200 ºC, the enhancement in the D band intensity compared to the G band is observed which signifies the creation of defects and distortion of SP2 domains after reduction[41]. Raman spectra of Zn-BDC and its composite with thermally reduced graphene oxide are shown in Fig. 4b. Raman Spectra of Zn-BDC match with the previously reported data [42]. The strong peak at 1435 cm−1 in Zn-BDC is attributed to the existence of Zn–O bonding between the Zn metal ion and linker, which shows the successful synthesis of Zn-BDC. The Raman spectra of Zn-BDC@rGO composite consists of the D and G band of rGO at 1350 cm−1 and 1598 cm−1, and the appearance of the Zn-BDC in the composite can be confirmed by the presence of a small signal at 92 cm−1 and 430 cm−1.
Raman mapping was performed by step-by-step moving sample using a motorized X–Y stage under the microscope, attached to the spectrometer to analyze the composition and distribution of Zn-BDC and rGO in the Zn-BDC@rGO composite, and the mapping image is shown in Fig. 4c. The red color indicates the presence of rGO, whereas the green color shows the distribution of Zn-BDC crystals. These results confirm the uniform distribution of Zn-BDC and rGO sheets.
3.5 Thermogravimetric (TGA) analysis
The GO, Zn-BDC, and Zn-BDC@rGO composite were characterized by thermogravimetric analysis in a nitrogen environment from 26 to 800 ºC, as depicted in Fig. 5. The four weight losses are observed in the GO. The first weight loss near 108 ºC represents eliminating absorbed water species. The weight losses near 188 ºC and 224 ºC are due to the breakdown of stable oxygen functional groups, and hence, near 200 ºC, GO gets partially reduced. The fourth weight loss between 440 and 495 ºC is due to the collapse of the graphene sheet[43]. The TGA results of Zn-BDC and its composite with rGO are nearly identical. The first weight loss between 85 to 120 ºC is attributed to the abolition of atmospheric water content. The second weight loss within 213–270 ºC is ascribed to the abolition of N, N-dimethylformamide. The third weight loss between 290 and 430 ºC is ascribed to the partial breakdown of Zn-BDC. The fourth weight loss between 560 and 594 ºC shows the complete collapse of Zn-BDC structure and formation of ZnO, H2O, CO2 as biproducts [44].
3.6 Atomic force microscopy
The 3D AFM images of the GO, rGO, Zn-BDC, and Zn-BDC@rGO composite are depicted in Fig. 6a–d, respectively. The average surface area of rGO is increased compared to the GO due to the exfoliation of the graphene sheet. Also, the root mean square roughness has increased while transforming from GO to partially reduced GO. The porous structure of the Zn-BDC can be confirmed from the images. When combined with rGO, the material’s porosity decreases because rGO sheets must have blocked some pores of Zn-BDC. Still, the surface area has increased from geometric area 9.00–14.2020 µm2 which is crucial for good sensitivity because the sensors sensitivity very much depends on the roughness of the sensing film. It is reported that the materials charge transfer capabilities highly rely on the materials morphology and surface-to-volume ratio [45, 46].
3.6.1 Brunauer–Emmett–Teller (BET) analysis
As depicted in Fig. 7a, b N2 adsorption–desorption isotherm and Barrett–Joyner–Halenda (BJH) pore size distribution of Zn-BDC and Zn-BDC@rGO show typical type-I adsorprption-desorption isotherm, which confirms the microporous nature [47]. All N2 adsorption–desorption measurements were taken at 77 K in liquid nitrogen. Langmuir and BET surface area for pure Zn-BDC was 137.63 m2/g and 142.75 m2/g, respectively. After forming the composite of Zn-BDC@rGO, the Langmuir and BET surface area was increased to 183.67 m2/g and 189.25 m2/g, respectively. Figure 7b shows the maximum pore size absorption in the range of 0.6–0.8 nm, and composite formation does not affect the pore distribution as expected.
3.7 Sensing performance
The shadow mask was used to fabricate the chemiresistive sensor electrodes on the glass substrate. Zn-BDC@rGO composite was drop casted on thermally coated copper electrodes having a gap of 100 µm on a glass substrate, and carbon monoxide, ammonia, and sulfur dioxide were tested using chemiresistive sensing modality. The sensors’ current–voltage characteristics are studied using the linear voltage sweep technique (Fig. 8a). It is observed that GO has very low conductivity. Zn-BDC MOF shows almost insulating in nature with the resistance 100 kΩ and 1 M, respectively, but when we reduced GO thermally its resistance decreased to 1 kΩ. In contrast, the S-shape nature of the Zn-BDC@rGO composite with a resistance of 200 kΩ confirms the semiconducting behavior. The chemiresistive sensing experiment was performed on a dynamic gas sensing setup. Alicat mass flow controllers were used to control the gas concentration and flow rate. The semiconductor parameter analyzer (Keithley-4200A) was used to measure the changes in resistance concerning shifts with time and gases concentrations. Response time and recovery time are the key parameters for the evolution of sensing performance. Figure 8b shows the real-time sensing graph. The fabricated sensor selectively discriminates among NH3, CO, and SO2 gases at 20 ppm, which is well below the PEL suggested by OSHA, USA with a response time/ recovery time of 60 s/120 s, respectively. Figure 8c shows the good linear dependency between resistance and analyte concentration for NH3, CO, and SO2 gases with very low deviation with the relative correlation coefficient (R2) values of 0.98447, 0.97875, and 0.98482, respectively. The consistent behavior of the sensor was analyzed by recording the sensing response for NH3, CO, and SO2 at 60 ppm for 30 days (Fig. 8d). Each measurement was taken after every five days by stabilizing the baseline, and the result shows the device is stable up to 30 days for all the tested gases, i.e., NH3, CO, and SO2. Principal component analysis (PCA) is an effectual technic to study the discriminating capability of the sensor toward different analytes. This technique reduces the dimensionality of data of interest to the principal component by decomposing the original data matrix into a coordinate base [48, 49]. As shown in Fig. 8e, principal component analysis (PCA) plot confirms that the sensor has discriminated among three gases, i.e., NH3, CO, and SO2.
The probable mechanism of the sensing is the synergetic effect between Zn-BDC and rGO. Since Zn-BDC has interesting properties like porous structure, high surface area, and catalytic behavior, it gives a good platform for gas absorption, but its application in chemiresistive sensing is limited because of its low electrical conductivity. Epoxy group present in the partially reduced graphene oxide replaces oxygen atom in the ZnO4 tetrahedra and forms a heterojunction between Zn-BDC and rGO. Hence, incorporation of rGO into Zn-BDC increases the material conductivity and catalytic activity [34, 50]. Which enhances the charge transform capabilities and leads to a change in electrical conductivity when exposed to the analyte. Camille Petit et.al. have reported that the synergetic effect between Zn-BDC and graphene increase the ammonia uptake capacity and ammonia react with zinc oxide tetrahedra via hydrogen bonding. Hence, Zn-BDC@rGO composite shows highest selectivity toward ammonia [37]. Table 1 shows the comparison of some of the MOF and graphene composite explored for the sensing application to date.
4 Conclusions
The sensor was fabricated by combining the uniqueness of the graphene layer and Zn-BDC and tested for chemiresistive sensing modality at room temperature to detect NH3, CO, and SO2 gases as fabricated sensors show clear discrimination among the NH3, CO, and SO2 gases. The LOD of the Zn-BDC@rGO sensor is 20 ppm which is far below the permissible exposure limit (PEL) suggested by the OSHA, USA, for CO and NH3 and very close to the PEL level of the SO2. The sensor has good response and recovery times, i.e., 60 s and 120 s, respectively. The sensor exhibits good stability and sensitivity, too. Principal component analysis has confirmed that the sensor has high discrimination capability among NH3, CO, and SO2. Hence, it can be used in sensing gadgets for real-time pollution monitoring.
Data Availability
Experimental data can be made available on request.
References
S. Bhandarkar, Vehicular pollution, their effect on human health and mitigation measures. Veh. Eng 1, 33–40 (2013)
B.R.G. Mabato, Y. Lyu, Y. Ji, Y.J. Li, D.D. Huang, X. Li, T. Nah, C.H. Lam, C.K. Chan, Aqueous secondary organic aerosol formation from the direct photosensitized oxidation of vanillin in the absence and presence of ammonium nitrate. Atmos. Chem. Phys. 22, 273–293 (2022). https://doi.org/10.5194/acp-22-273-2022
R.D. Brook, S. Rajagopalan, C.A. Pope III., J.R. Brook, A. Bhatnagar, A.V. Diez-Roux, F. Holguin, Y. Hong, R.V. Luepker, M.A. Mittleman, Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation 121, 2331–2378 (2010). https://doi.org/10.1161/CIR.0b013e3181dbece1
A. Ghorani-Azam, B. Riahi-Zanjani, M. Balali-Mood, Effects of air pollution on human health and practical measures for prevention in Iran. J. Res. Med. Sci. (2016). https://doi.org/10.4103/1735-1995.189646
S. Dhital, D. Rupakheti, Bibliometric analysis of global research on air pollution and human health: 1998–2017. Environ. Sci. Pollut. Res.Pollut. Res. 26, 13103–13114 (2019). https://doi.org/10.1007/s11356-019-04482-x
D.J. Nowak, S. Hirabayashi, M. Doyle, M. McGovern, J. Pasher, Air pollution removal by urban forests in Canada and its effect on air quality and human health. Urban For. Urban Green. 29, 40–48 (2018). https://doi.org/10.1016/j.ufug.2017.10.019
H. Apsimon, M. Kruse-Plass, The role of ammonia as an atmospheric pollutant (Elsevier Applied Science Publications, London, 1991)
S. Pandey, G.K. Goswami, K.K. Nanda, Nanocomposite based flexible ultrasensitive resistive gas sensor for chemical reactions studies. Sci. Rep. 3, 1–6 (2013)
V.R. Ivanova, The anthropogenic air pollution and human health. J. IMAB Annu. Proc. Sci. Papers 26, 3057–3062 (2020)
A. Abelsohn, M.D. Sanborn, B.J. Jessiman, E. Weir, Identifying and managing adverse environmental health effects: 6. Carbon monoxide poisoning. CMAJ 166, 1685–1690 (2002)
K. Datta, P. Ghosh, M. More, M. Shirsat, A. Mulchandani, Controlled functionalization of single-walled carbon nanotubes for enhanced ammonia sensing: a comparative study. J. Phys. D Appl. Phys. 45, 355305 (2012). https://doi.org/10.1088/0022-3727/45/35/355305
H.Y. Mohammed, M.A. Farea, P.W. Sayyad, N.N. Ingle, T. Al-Gahouari, M.M. Mahadik, G.A. Bodkhe, S.M. Shirsat, M.D. Shirsat, Selective and sensitive chemiresistive sensors based on polyaniline/graphene oxide nanocomposite: a cost-effective approach. J. Sci. Adv. Mater. Devices 7, 100391 (2022). https://doi.org/10.1016/j.jsamd.2021.08.004
J.L. Johnson, A. Behnam, Y. An, S. Pearton, A. Ural, Experimental study of graphitic nanoribbon films for ammonia sensing. J. Appl. Phys. 109, 124301 (2011). https://doi.org/10.1063/1.3597635
C. Wang, S. Lei, X. Li, S. Guo, P. Cui, X. Wei, W. Liu, H. Liu, A reduced GO-graphene hybrid gas sensor for ultra-low concentration ammonia detection. Sensors 18, 3147 (2018). https://doi.org/10.3390/s18093147
M.M. Sivalingam, K. Balasubramanian, Influence of the concentration of reducing agent on gold nanoparticles decorated reduced graphene oxide and its ammonia sensing performance. Appl. Phys. A 123, 281 (2017). https://doi.org/10.1007/s00339-017-0910-9
J. Min Lee, H. Yong Jeong, K. Jin Choi, W. Il Park, Metal/graphene sheets as p-type transparent conducting electrodes in GaN light emitting diodes. Appl. Phys. Lett. 99, 041115 (2011). https://doi.org/10.1063/1.3595941
S.-Y. Lee, S.-J. Park, Comprehensive review on synthesis and adsorption behaviors of graphene-based materials. Carbon Lett. 13, 73–87 (2012). https://doi.org/10.5714/CL.2012.13.2.073
P. Cao, Y. Cai, D. Pawar, S. Navale, C.N. Rao, S. Han, W. Xu, M. Fang, X. Liu, Y. Zeng, Down to ppb level NO2 detection by ZnO/rGO heterojunction based chemiresistive sensors. Chem. Eng. J. 401, 125491 (2020)
R.D. Martínez-Orozco, R. Antaño-López, V. Rodríguez-González, Hydrogen-gas sensors based on graphene functionalized palladium nanoparticles: impedance response as a valuable sensor. New J. Chem. 39, 8044–8054 (2015). https://doi.org/10.1039/C5NJ01673H
D. Wu, P.-F. Zhang, G.-P. Yang, L. Hou, W.-Y. Zhang, Y.-F. Han, P. Liu, Y.-Y. Wang, Supramolecular control of MOF pore properties for the tailored guest adsorption/separation applications. Coord. Chem. Rev. 434, 213709 (2021). https://doi.org/10.1016/j.ccr.2020.213709
L.E. Kreno, K. Leong, O.K. Farha, M. Allendorf, R.P. Van Duyne, J.T. Hupp, Metal–organic framework materials as chemical sensors. Chem. Rev. 112, 1105–1125 (2012). https://doi.org/10.1021/cr200324t
F.Y. Yi, D. Chen, M.K. Wu, L. Han, H.L. Jiang, Chemical sensors based on metal–organic frameworks. ChemPlusChem 81, 675–690 (2016). https://doi.org/10.1002/cplu.201600137
F.G. Moscoso, J. Almeida, A. Sousaraei, T. Lopes-Costa, A.M. Silva, J. Cabanillas-Gonzalez, L. Cunha-Silva, J.M. Pedrosa, Luminescent MOF crystals embedded in PMMA/PDMS transparent films as effective NO2 gas sensors. Mol. Syst. Design Eng. 5, 1048–1056 (2020). https://doi.org/10.1039/C9ME00164F
J. Zhang, F. Liu, J. Gan, Y. Cui, B. Li, Y. Yang, G. Qian, Metal-organic framework film for fluorescence turn-on H2S gas sensing and anti-counterfeiting patterns. Sci. China Mater. 62, 1445–1453 (2019). https://doi.org/10.1007/s40843-019-9457-5
P. Davydovskaya, R. Pohle, A. Tawil, M. Fleischer, Work function based gas sensing with Cu-BTC metal-organic framework for selective aldehyde detection. Sens. Actuators B Chem. 187, 142–146 (2013). https://doi.org/10.1016/j.snb.2012.10.023
L. Wang, Metal-organic frameworks for QCM-based gas sensors: a review. Sens. Actuators, A 307, 111984 (2020). https://doi.org/10.1016/j.sna.2020.111984
N.A. Travlou, K. Singh, E. Rodriguez-Castellon, T.J. Bandosz, Cu–BTC MOF–graphene-based hybrid materials as low concentration ammonia sensors. J. Mater. Chem. A 3, 11417–11429 (2015). https://doi.org/10.1039/C5TA01738F
S. Cho, C. Park, M. Jeon, J.H. Lee, O. Kwon, S. Seong, J. Kim, I.-D. Kim, H.R. Moon, Interface-sensitized chemiresistor: Integrated conductive and porous metal-organic frameworks. Chem. Eng. J. 449, 137780 (2022). https://doi.org/10.1016/j.cej.2022.137780
M. Ko, A. Aykanat, M.K. Smith, K.A. Mirica, Drawing sensors with ball-milled blends of metal-organic frameworks and graphite. Sensors 17, 2192 (2017). https://doi.org/10.3390/s17102192
M.G. Campbell, S.F. Liu, T.M. Swager, M. Dinca, Chemiresistive sensor arrays from conductive 2D metal–organic frameworks. J. Am. Chem. Soc. 137, 13780–13783 (2015)
M.S. Yao, W.X. Tang, G.E. Wang, B. Nath, G. Xu, MOF thin film-coated metal oxide nanowire array: significantly improved chemiresistor sensor performance. Adv. Mater. 28, 5229–5234 (2016)
M.E. DMello, N.G. Sundaram, A. Singh, A.K. Singh, S.B. Kalidindi, An amine functionalized zirconium metal–organic framework as an effective chemiresistive sensor for acidic gases. Chem. Commun.Commun. 55, 349–352 (2019)
X. Yang, T. Fraser, D. Myat, S. Smart, J. Zhang, J.C. Diniz da Costa, A. Liubinas, M. Duke, A pervaporation study of ammonia solutions using molecular sieve silica membranes. Membranes 4, 40–54 (2014). https://doi.org/10.3390/membranes4010040
C. Petit, T.J. Bandosz, MOF–graphite oxide composites: combining the uniqueness of graphene layers and metal–organic frameworks. Adv. Mater. 21, 4753–4757 (2009). https://doi.org/10.1002/adma.200901581
L. Shahriary, A.A. Athawale, Graphene oxide synthesized by using modified hummers approach. Int. J. Renew. Energy Environ. Eng 2, 58–63 (2014)
M.S. More, G.A. Bodkhe, N.N. Ingle, F. Singh, M.-L. Tsai, M. Kim, M.D. Shirsat, Metal-organic framework (MOF)/reduced graphene oxide (rGO) composite for high performance CO sensor. Solid-State Electron. 204, 108638 (2023)
C. Petit, T.J. Bandosz, Enhanced adsorption of ammonia on metal-organic framework/graphite oxide composites: analysis of surface interactions. Adv. Funct. Mater.Funct. Mater. 20, 111–118 (2010)
S.N. Alam, N. Sharma, L. Kumar, Synthesis of graphene oxide (GO) by modified hummers method and its thermal reduction to obtain reduced graphene oxide (rGO). Graphene 6, 1–18 (2017)
T. Noor, N. Zaman, H. Nasir, N. Iqbal, Z. Hussain, Electro catalytic study of NiO-MOF/rGO composites for methanol oxidation reaction. Electrochim. Acta. Acta 307, 1–12 (2019)
A.T. Habte, D.W. Ayele, Synthesis and characterization of reduced graphene oxide (rGO) started from graphene oxide (GO) using the tour method with different parameters. Adv. Mater. Sci. Eng. (2019). https://doi.org/10.1155/2019/5058163
K. Krishnamoorthy, M. Veerapandian, R. Mohan, S.-J. Kim, Investigation of Raman and photoluminescence studies of reduced graphene oxide sheets. Appl. Phys. A 106, 501–506 (2012). https://doi.org/10.1007/s00339-011-6720-6
N.A. Rodríguez, R. Parra, M.A. Grela, Structural characterization, optical properties and photocatalytic activity of MOF-5 and its hydrolysis products: implications on their excitation mechanism. RSC Adv. 5, 73112–73118 (2015). https://doi.org/10.1039/C5RA11182J
G. Kumar, D.T. Masram, Sustainable synthesis of MOF-5@ GO nanocomposites for efficient removal of rhodamine B from water. ACS Omega 6, 9587–9599 (2021). https://doi.org/10.1021/acsomega.1c00143
J. Yuan, W. Liu, X. Zhang, Y. Zhang, W. Yang, W. Lai, X. Li, J. Zhang, X. Li, MOF derived ZnSe–FeSe2/RGO Nanocomposites with enhanced sodium/potassium storage. J. Power. Sources 455, 227937 (2020). https://doi.org/10.1016/j.jpowsour.2020.227937
A. Benoudjit, M.M. Bader, W.W.A.W. Salim, Study of electropolymerized PEDOT: PSS transducers for application as electrochemical sensors in aqueous media. Sens. Bio-sens. Res. 17, 18–24 (2018). https://doi.org/10.1016/j.sbsr.2018.01.001
P.W. Sayyad, S.S. Khan, N.N. Ingle, G.A. Bodkhe, T. Al-Gahouari, M.M. Mahadik, S.M. Shirsat, M.D. Shirsat, Chemiresistive SO2 sensor: graphene oxide (GO) anchored poly (3, 4-ethylenedioxythiophene): poly (4styrenesulfonate)(PEDOT: PSS). Appl. Phys. A 126, 1–8 (2020). https://doi.org/10.1007/s00339-020-04053-9
Y. Zhu, Y.-M. Wang, P. Liu, Y.-L. Wu, W. Wei, C.-K. Xia, J.-M. Xie, Cage-like pores of a metal–organic framework for separations and encapsulation of Pd nanoparticles for efficient catalysis. New J. Chem. 39, 2669–2674 (2015)
S.M. Shirsat, G.A. Bodkhe, M.M. Sonawane, B.W. Gawali, M.D. Shirsat, Multivariate analysis of a cobalt octaethyl porphyrin-functionalized SWNT microsensor device for selective and simultaneous detection of multiple analytes. J. Electron. Mater. 50, 5780–5787 (2021). https://doi.org/10.1007/s11664-021-09111-3
T. Alizadeh, L.H. Soltani, Reduced graphene oxide-based gas sensor array for pattern recognition of DMMP vapor. Sens. Actuators B Chem. 234, 361–370 (2016). https://doi.org/10.1016/j.snb.2016.04.165
C. Petit, T.J. Bandosz, MOF–graphite oxide nanocomposites: surface characterization and evaluation as adsorbents of ammonia. J. Mater. Chem. 19, 6521–6528 (2009)
M.K. Smith, K.A. Mirica, Self-organized frameworks on textiles (SOFT): conductive fabrics for simultaneous sensing, capture, and filtration of gases. J. Am. Chem. Soc. 139, 16759–16767 (2017). https://doi.org/10.1021/jacs.7b08840
X. Huang, N. Hu, L. Zhang, L. Wei, H. Wei, Y. Zhang, The NH3 sensing properties of gas sensors based on aniline reduced graphene oxide. Synth. Metals 185, 25–30 (2013). https://doi.org/10.1016/j.synthmet.2013.09.034
Y. Seekaew, S. Lokavee, D. Phokharatkul, A. Wisitsoraat, T. Kerdcharoen, C. Wongchoosuk, Low-cost and flexible printed graphene–PEDOT: PSS gas sensor for ammonia detection. Org. Electron. 15, 2971–2981 (2014). https://doi.org/10.1016/j.orgel.2014.08.044
X. Tang, D. Lahem, J.-P. Raskin, P. Gérard, X. Geng, N. André, M. Debliquy, A fast and room-temperature operation ammonia sensor based on compound of graphene with polypyrrole. IEEE Sens. J. 18, 9088–9096 (2018). https://doi.org/10.1109/JSEN.2018.2869203
T.T. Tung, M.T. Tran, J.-F. Feller, M. Castro, T. Van Ngo, K. Hassan, M.J. Nine, D. Losic, Graphene and metal organic frameworks (MOFs) hybridization for tunable chemoresistive sensors for detection of volatile organic compounds (VOCs) biomarkers. Carbon 159, 333–344 (2020). https://doi.org/10.1016/j.carbon.2019.12.010
P.-G. Su, S.-L. Peng, Fabrication and NO2 gas-sensing properties of reduced graphene oxide/WO3 nanocomposite films. Talanta 132, 398–405 (2015). https://doi.org/10.1016/j.talanta.2014.09.034
Acknowledgements
The authors extend their sincere thanks to Inter-University Accelerator Center (IUAC), New Delhi, India (UFR no. 69330), UGC—DAE CSR, Indore (Project No. CRS/2021-22/ 01/456 dated March 30, 2022), DST—SERB, New Delhi (Project No. EEQ/2017/000645), UGC-SAP Programme (F.530/16/DRS-I/2016 (SAP-II) Dt.16-04-2016), DST-FIST (Project No. SR/FST/PSI-210/2016(c) dtd. 16/12/2016) and Rashtriya Uchachatar Shiksha Abhiyan (RUSA), Government of Maharashtra for providing financial support. Mahendra D, Shirsat gratefully acknowledges the Slovak Academic Information Agency (SAIA) and Department of Nuclear Physics and Biophysics, Faculty of Mathematics, Physics and Informatics, Comenius University, Bratislava, Slovak Republic, for the sanction of scholarship under the framework of National Scholarship Program (NSP) of Slovak Republic.
Funding
Open access funding provided by The Ministry of Education, Science, Research and Sport of the Slovak Republic in cooperation with Centre for Scientific and Technical Information of the Slovak Republic
Author information
Authors and Affiliations
Contributions
MSM conceptualization, investigation, methodology, data analysis, writing—original draft; GAB conceptualization, investigation, methodology, data analysis, writing—original draft; FS manuscript review and editing. MK review and editing, supervision, revisions. MDS conceptualization, supervision, writing—review and editing, project administration, funding acquisition.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
More, M.S., Bodkhe, G.A., Singh, F. et al. Metal–organic framework-reduced graphene oxide (Zn-BDC@rGO) composite for selective discrimination among ammonia, carbon monoxide, and sulfur dioxide. Appl. Phys. A 129, 828 (2023). https://doi.org/10.1007/s00339-023-07103-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-023-07103-0