Abstract
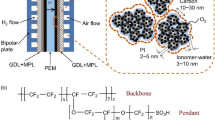
Proton Exchange Membrane (PEM) fuel cell electrodes with different ionomer contents were studied with various microscopic techniques. The morphology and surface potential were examined by Atomic Force Microscopy (AFM) and Kelvin Probe Microscopy (KPM), respectively. The particulate nature of the electrode was well displayed in the topography and phase images. The particle and pore size (Z) distributions showed the most frequent values at 30–40 nm and 20–30 nm, respectively. The particle size corresponds to the size of the carbon support for the platinum catalyst. Catalyst agglomeration was observed in high ionomer content electrodes. The surface potential images showed distinct difference to the topography images. The overall grain size was seen to increase, the pore volume to decrease, the surface roughness to decrease, and the surface potential variation to increase with the increase of ionomer content in the catalyst layer. Transmission electron microscopy (TEM) was carried out on selective electrodes to provide additional information and confirmed with the AFM results. Cyclic voltammetry (CV) showed that the electrode containing 30 wt.% ionomer has maximum catalyst utilization.
Similar content being viewed by others
References
C. Stone, A.E. Morrison, Solid State Ion. 152, 1–13 (2002)
B. Smitha, S. Sridhar, A.A. Khan, J. Membr. Sci. 259, 10–26 (2005)
H. Nakajima, S. Nomura, T. Sugimoto et al., J. Electrochem. Soc. 149(8), A953–A959 (2002)
Q.F. Li, R.H. He, J.O. Jensen et al., Chem. Mater. 15(26), 4896–4915 (2003)
R. Souzy, B. Ameduri, Prog. Polym. Sci. 30(6), 644–687 (2005)
T.R. Ralph, G.A. Hards, J.E. Keating et al., J. Electrochem. Soc. 144(11), 3845–3857 (1997)
S.D. Thompson, L.R. Jordan, M. Forsyth, Electrochim. Acta 46(10–11), 1657–1663 (2001)
Z.R. Ismagilov, M.A. Kerzhentsev, N.V. Shikina et al., Catal. Today 102, 58–66 (2005)
J.H. Wee, K.Y. Lee, S.H. Kim, J. Power Sources 165(2), 667–677 (2007)
O. Antoine, Y. Bultel, R. Durand, J. Electroanal. Chem. 499(1), 85–94 (2001)
B. Wang, J. Power Sources 152(1), 1–15 (2005)
R.G. Gonzalez-Huerta, J.A. Chavez-Carvayar, O. Solorza-Feria, J. Power Sources 153(1), 11–17 (2006)
S. Litster, G. McLean, J. Power Sources 130(1–2), 61–76 (2004)
N.P. Brandon, S. Skinner, B.C.H. Steele, Annu. Rev. Mater. Res. 33, 183–213 (2003)
X.L. Cheng, B.L. Yi, M. Han et al., J. Power Sources 79(1), 75–81 (1999)
A. Taniguchi, T. Akita, K. Yasuda et al., J. Power Sources 130(1–2), 42–49 (2004)
J. Xie, D.L. Wood, D.M. Wayne et al., J. Electrochem. Soc. 152(1), A104–A113 (2005)
H. Ghassemi, J.E. McGrath, T.A. Zawodzinski, Polymer 47(11), 4132–4139 (2006)
H. Inoue, H. Daiguji, E. Hihara, JSME Int. J., Ser. B 47(2), 228–234 (2004)
J. Zhang, G.P. Yin, Z.B. Wang et al., J. Power Sources 160(2), 1035–1040 (2006)
M. Sogaard, M. Odgaard, M.E. Skou, Solid State Ion. 145(1–4), 31–35 (2001)
S. Ma, Q. Chen, F.H. Jørgensen et al., Solid State Ion. 178(29–30), 1568–1575 (2007)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ma, S., Solterbeck, CH., Odgaard, M. et al. Microscopy studies on pronton exchange membrane fuel cell electrodes with different ionomer contents. Appl. Phys. A 96, 581–589 (2009). https://doi.org/10.1007/s00339-008-5050-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00339-008-5050-9