Abstract
Invertivorous fishes are key middle-order consumers that connect energy flows across different trophic levels. However, the potential for distinct functional roles to exist within this trophic guild has not been satisfactorily explored to date, meaning that current assessments of ecosystem resilience are likely to over-estimate the level of functional redundancy within a given invertivorous fish assembly. Our study examined the foraging behaviour and microhabitat preferences of invertivorous fish communities within the productive canopy macroalgal meadows of Ningaloo Marine Park, Western Australia. Our aim was to identify foraging specialisations that could yield distinct functional roles for species belonging to the guild. We found that invertivorous fishes at this location were chiefly represented by species belonging to the Labridae, Lethrinidae and Mullidae families. Individual species demonstrated strong preferences for foraging within specific microhabitat types, suggesting that the guild can be grouped into three categories of foraging specialists: ‘canopy forager’, ‘generalist’ and ‘abiotic forager’. Our results highlight subtle niche partitioning of foraging microhabitats within the trophic guild of invertivorous fishes associated with tropical macroalgal meadows. Moreover, this partitioning is consistent across seasons, despite significant fluctuations in canopy structure and biomass. The resulting refinement of foraging specialisations allows us to identify the functional roles of invertivorous fishes and afford greater protection to individual species that might otherwise be considered functionally redundant. Our results will help to inform knowledge of the functional impact of particular species and their ecological specialisations and improve our understanding of trophic flows in marine food webs for appropriate management and conservation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In marine ecosystems, invertivorous fishes (i.e. species that primarily feed on invertebrates) can represent one of the dominant feeding guilds (Longo et al. 2019; Parravicini et al. 2020). For example, nearly 70% of fish species on the Great Barrier Reef, Australia, have been classified as feeding predominantly on invertebrates (Randall et al. 1997; Kramer et al. 2015; Froese and Pauly 2021). The overall guild of invertivorous fishes comprises a diverse range of families, many of which are commonly fished (Sumner et al. 2002; Fulton et al. 2020; Froese and Pauly 2021). Invertivorous fishes have been shown to connect energy flows between primary producers and higher-order consumers, as they are predators that feed on invertebrates supported by primary production, but are themselves also the target prey of mesopredatory piscivores and apex predators (Edgar and Aoki 1993; Newcombe and Taylor 2010; Ashworth et al. 2014; Bergström et al. 2016; Froese and Pauly 2021). The trophic links facilitated by invertivorous fishes therefore underpin fundamental processes of marine ecosystem functioning that can influence global recreational and commercial fishery stocks (Lewis and Anderson 2012; Fulton et al. 2020; Froese and Pauly 2021).
The identification of essential habitats used by fishes is a critical step in the process of ecosystem-based fishery management of commercially and recreationally important species (Beck et al. 2001; Thrush and Dayton 2010; Wilson et al. 2017). Foraging behaviour is a key aspect of habitat use by fishes and can be documented as preferences relating to particular microhabitat types (Krajewski and Floeter 2011; Fulton et al. 2016; Kramer et al. 2016). For example, strong dependency on preferred microhabitats can lead to dramatic changes in the temporal and spatial dynamics of fish populations or communities following shifts in the availability of those preferred microhabitats. Local extinction of some coral reef fish species can occur when the corals they exclusively prey on are no longer available (Westmacott et al. 2000; Pratchett et al. 2018), and carnivorous fishes can also be vulnerable to the loss of preferred foraging microhabitats (Munday 2004; Wilson et al. 2008a; Wenger et al. 2018). In addition to that, documenting species’ microhabitat specialisations is fundamental to defining their ‘ecosystem function’ in terms of positioning along the feeding niche resource axis (MacArthur 1958; Hutchinson 1959). The monitoring and management of marine ecosystems is increasingly based around the protection of critical functional groups (Green and Bellwood 2009; Graham et al. 2013; Villéger et al. 2017), where a species’ ecosystem function is defined based on its ecological traits (Bellwood et al. 2019). This approach has led to the recognition that members of particular trophic groupings are not ecological equivalents. For example, on coral reefs, the group of fishes previously defined collectively under the trophic status of ‘herbivore’ has now been carved up into many different ecosystem functions, based on factors such as mode of feeding (‘scrapers’ versus ‘excavators’ (Bellwood and Choat 1990), ‘grazers’ versus ‘browsers’ (Choat et al. 2002; Fox and Bellwood 2008; Green and Bellwood 2009; Hoey and Bellwood 2009) and ‘croppers’ (Green and Bellwood 2009) or on microhabitat preferences (‘crevice feeders’ versus ‘open matrix feeders’ (Fox and Bellwood 2013; Brandl and Bellwood 2014), ‘leaf-biters’ versus ‘thallus biters’ (Streit et al. 2015). This process of trophic group refinement into functional groupings can help to provide a more accurate assessment of ecosystem resilience, by identifying species that perform unique ecosystem functions and yielding a more conservative estimate of the level of functional redundancy associated with the biodiversity present within a particular community structure (Cheal et al. 2010, 2012; Rasher et al. 2013).
Within tropical seascapes, invertivorous fishes have the potential to exploit heterogeneous microhabitats to feed on epifaunal and infaunal invertebrates (Kwak et al. 2015; van Lier et al. 2018; Sambrook et al. 2019). Although strong microhabitat preferences of tropical invertivorous fishes driven by specific dietary targets have previously been documented (Lukoschek and McCormick 2001; Wilson et al. 2008b; Wenger et al. 2018), most studies to date on the microhabitat utilisation of these invertivorous fishes either have focused on well-studied habitats, such as coral reefs, or have looked only at the microhabitat preferences of a single species of invertivore (Layton and Fulton 2014; Brandl et al. 2015; Kramer et al. 2016; Wenger et al. 2018). Current knowledge of how the overall collective trophic grouping of invertivorous fish species demonstrate niche partitioning of their foraging microhabitats in non-reef habitats is limited. Theory would predict, however, that within the overall invertivore assemblage, individual species exploit different portions of the habitat space, exhibiting niche partitioning at a finer scale (Floeter et al. 2007; Berkström et al. 2012; Asher et al. 2017; Brandl et al. 2020). Knowledge of these microhabitat specialisations is therefore an important first step in defining the ecosystem function of species within the invertivore trophic guild.
One of the most common and productive non-reef habitats within tropical seascapes is macroalgal meadows, comprised of canopy-forming macroalgae (Tano et al. 2016; Fulton et al. 2020). These macroalgal meadows can extend over significant portions of shallow tropical marine habitats (estimated between 16 and 46% of some shallow coastal areas, Fulton et al. 2019), forming complex habitat structures and contributing a large amount of areal primary production. This primary production supports communities of epifaunal invertebrates, which, in turn, provide nutrition for invertivorous fishes (Edgar and Aoki 1993; Wenger et al. 2018). Recent studies have highlighted the fact that these macroalgal meadows and their associated epifaunal communities are important foraging grounds for invertivorous fishes (Chaves et al. 2013; Chen et al. 2020). Macroalgal meadows can, however, exhibit strong temporal shifts in canopy size (in terms of either overall biomass or the length of macroalgal thalli). In tropical regions, one of the typical temporal canopy shifts is canopy growth in summer and detachment in winter (Leite and Turra 2003; Wong and Phang 2004; Lefevre and Bellwood 2010; Fulton et al. 2014). Seasonal fluctuations in macroalgal canopy size therefore influence the availability of habitat, impacting the abundance and availability of associated epifaunal invertebrate communities (Taylor 1998; Leite and Turra 2003; Ba-Akdah et al. 2016), and the invertivorous fishes that prey on epifauna (Edgar and Aoki 1993; Fulton et al. 2019; Froese and Pauly 2021). However, we lack a basic understanding of the microhabitat preferences of macroalgal-associated invertivorous fishes while foraging within macroalgal meadows, and how such foraging microhabitat preferences of individual invertivorous species might respond to seasonal fluctuations in canopy size that are likely to affect the availability of epifaunal prey. This hinders our ability to understand spatial niche partitioning within this trophic group and the potential for within-group functional complementarity versus redundancy. The aims of this study were therefore: (1) to document the foraging microhabitat preferences of the dominant invertivorous fish species within macroalgal meadows of the world heritage Ningaloo Marine Park, Western Australia, and determine the potential for functional complementarity based on the microhabitat resource axis within this trophic group and (2) to examine how these microhabitat preferences respond to seasonal shifts in macroalgal canopy structure (summer to winter).
Methods
Study region
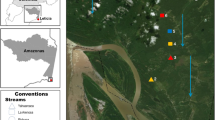
This study was conducted within the Maud Recreation Region of Ningaloo Marine Park near Coral Bay, situated in the north-west of Western Australia (Fig. 1). As Australia’s largest fringing coral reef (~ 290 km long), shallow waters (3–5 m depth) in this area are dominated by canopy-forming fucoids chiefly belonging to the genus Sargassum. These canopies form extensive macroalgal meadow patches covering over 300 km2, which exhibit strong seasonal fluctuations in macroalgal biomass (Kobryn et al. 2013; Fulton et al. 2014; van Lier et al. 2018; Chen et al. 2020). A total of eight Sargassum meadow patches (size: 28,893 ± 11,627 m2) were surveyed during late austral summer (February–March) 2018 to confirm the presence of invertivorous fishes and determine habitat composition (Fig. 1). Only four of these Sargassum meadow patches were reinvestigated during the austral winter (August–September) 2018 due to the dramatic seasonal decline of Sargassum canopy biomass (Fig. 1).
Habitat composition of macroalgal meadows
Habitat composition of each Sargassum meadow patch in summer and winter was documented via underwater visual censuses conducted by divers on SCUBA, following Lim et al. (2016). At each meadow patch, we haphazardly deployed six replicate 10-m transect tapes and recorded the distance along each transect (to the nearest 5 cm) occupied by three distinct habitat categories: (1) canopy macroalgae (leathery macrophytes with the canopy height can reach around 10–50 cm, and up to 1–2 m, e.g. Sargassum, Sargassopsis), (2) understory macroalgae (foliose macrophytes without canopies, occupying the floor of meadows, e.g. Lobophora, Dictyota, Padina) and (3) abiotic components (e.g. pavement, sand, dead coral, rubble). Converting these distances to a proportion of the 10-m transect length gave us a percentage composition of the three habitat types within each meadow.
Foraging microhabitat preferences of invertivorous fishes
The use of individual foraging microhabitats by species of invertivorous fishes was recorded using underwater visual observations by divers on SCUBA. At each meadow patch during summer and winter, at least three instantaneous focal surveys were conducted (following Fulton et al. 2001) over 8 days in summer and 5 days in winter. In brief, a single diver swam a random, non-overlapping path starting from the centre of each meadow patch out to the patch edge. Surveys commenced 5 min after the diver had reached the patch centre to allow for fish to acclimate to diver presence. For each invertivorous fish observed to show direct foraging behaviour (following Wenger et al. 2018), we recorded the species identity, total length (TL, to the nearest cm), foraging behaviour (searching/feeding) and microhabitat location (e.g. canopy macroalgae). Within these focal observations of direct foraging behaviour, ‘searching’ was strictly defined as the fish having its head inclined towards the particular microhabitat but without touching, while ‘feeding’ was defined by its mouth being in contact with the microhabitat. Subsequently, ‘searching’ and ‘feeding’ in each season were pooled together for further analysis. To avoid problems associated with inferences based on low sample sizes/sites, during summer, only invertivorous species represented by more than ten individuals per meadow patch and present on at least three meadow patches were included in subsequent analyses. During winter, we adjusted this rule to species represented by more than ten individuals per meadow patch and present on at least two of the four meadow patches surveyed. Foraging microhabitat preferences of each invertivorous fish species were determined using the electivity index formula of Vanderploeg and Scavia (1979):
where Ei* is the electivity for microhabitat category i, n is the number of microhabitat categories and Wi is the selective coefficient for microhabitat category i calculated as:
where ri is the proportional use of the microhabitat category i and pi is the proportional availability of the microhabitat category i. Values of electivity indices (Ei*) range from −1 to 1, with indication of avoidance (negative value), neutrality (Ei* = 0) and preference (positive value) for a particular microhabitat. Electivity index of each species was averaged across patches within a given season to determine the patterns of season-specific foraging microhabitat associations of individual invertivorous fishes in the Maud Recreation Region of Ningaloo Marine Park.
Results
Invertivorous fish communities
Based on 3207 individual foraging behaviour recorded (2538 in summer and 669 in winter, Supplemental Table S1), we observed a total of 36 invertivorous fish species foraging within macroalgal meadows in the summer (34 species) and winter (24 species). Of these 36 species, 12 species in summer and 5 in winter (Table 1) met our criterion for the analysis of foraging microhabitat preferences (Table 2). The majority of invertivorous fishes for whom we recorded foraging abundance (the number of individuals exhibiting foraging behaviour) belonged to the family Labridae (71% in summer, 75% in winter), followed by Lethrinidae (17% in summer, 10% in winter) and Mullidae (11% in summer, 15% in winter). The 12 most abundant species were as follows: (1) Labridae: Anampses geographicus, Cheilio inermis, Coris caudimacula, Halichoeres nebulosus, Pseudojuloides elongatus, Stethojulis bandanensis, Stethojulis interrupta and Thalassoma lunare; (2) Lethrinidae: Lethrinus atkinsoni and Lethrinus nebulosus; and (3) Mullidae: Parupeneus barberinoides and Parupeneus spilurus (Table 1). The number of species and/or the total number of foraging individuals of each species declined in winter, which changed the community composition (in terms of foraging abundance) at each meadow patch (Table 2).
Habitat availability and microhabitat use
Habitat composition in each meadow patch showed that canopy macroalgae was the dominant microhabitat in summer (Fig. 2a). In winter, patches shifted to be dominated by the abiotic component due to the dramatic seasonal decline in Sargassum canopy biomass (Fig. 2b). Invertivorous fishes used distinct microhabitats while foraging (Figs. 3, 4). During the summer, five invertivorous fishes (Anampses geographicus, Cheilio inermis, Coris caudimacula, Pseudojuloides elongatus and Thalassoma lunare) were observed foraging primarily within the canopy macroalgae compared with the understory macroalgae or abiotic components (Fig. 3a–e). The proportional use of canopy macroalgae was higher than its availability for these species, suggesting a strong foraging microhabitat preference for canopy macroalgae. The opposite pattern, which suggested a strong microhabitat preference for abiotic components, was found for Halichoeres nebulosus, Parupeneus barberinoides, Parupeneus spilurus, Stethojulis bandanensis and Stethojulis interrupta (Fig. 3h–l). Interestingly, these patterns of disproportionately using certain microhabitats were consistent for the four species (Cheilio inermis, Coris caudimacula, Halichoeres nebulosus and Parupeneus spilurus) observed in both seasons, with Thalassoma lunare as the only exception (Fig. 4).
Foraging microhabitat use by invertivorous fishes in summer, as indicated by the proportional use relative to the proportional availability of three microhabitat categories: a Anampses geographicus, b Cheilio inermis, c Coris caudimacula, d Pseudojuloides elongatus, e Thalassoma lunare, f Lethrinus atkinsoni, g Lethrinus nebulosus, h Halichoeres nebulosus, i Parupeneus barberinoides, j Parupeneus spilurus, k Stethojulis bandanensis and l Stethojulis interrupta
Foraging microhabitat preferences of invertivorous fishes
Electivity indices revealed that invertivorous fishes foraging within macroalgal meadows can be chiefly grouped into three categories of foraging specialisations: ‘canopy forager’, ‘generalist’ and ‘abiotic forager’ (Figs. 5, 6). ‘Canopy foragers’ (i.e. those fish that search for prey and feed within macroalgal canopies) were overwhelmingly represented by Labridae, specifically the species Anampses geographicus, Cheilio inermis, Coris caudimacula, Pseudojuloides elongatus and Thalassoma lunare in summer (Fig. 5) and Cheilio inermis and Coris caudimacula in winter (Fig. 6). Notably, this positive preference for canopy macroalgae was consistent across seasons, even in winter when there was significantly lower availability of macroalgal canopy (Figs. 5, 6). The only exception was Thalassoma lunare, which showed foraging preferences for both canopy and understory macroalgae in winter, indicating a shift from canopy forager to being a generalist when canopy macroalgae were less available (Fig. 6). Species belonging to the family Mullidae (Parupeneus barberinoides and Parupeneus spilurus in summer; Parupeneus spilurus in winter) as well as the labrid species (Halichoeres nebulosus, Stethojulis bandanensis and Stethojulis interrupta in summer; Halichoeres nebulosus in winter) were found to be ‘abiotic foragers’ (fish that search for prey and feed on pavement, sand, dead coral, rubble) (Figs. 5, 6). Finally, during summer, species belonging to the family Lethrinidae (Lethrinus atkinsoni and Lethrinus nebulosus) were found to be ‘generalists’, showing a positive electivity for foraging within both canopy macroalgae and on abiotic substrates (Fig. 5). However, none of these lethrinid generalist species were observed in sufficient number during the winter surveys. Interestingly, almost all the fish species (excluding Thalassoma lunare in winter) in this study showed a strong tendency to avoid foraging within understory macroalgae, despite its high availability within the macroalgal patches (Figs. 5, 6).
Foraging preferences of common invertivorous fishes for three microhabitat categories (canopy macroalgae, understory macroalgae and abiotic components) averaged (± standard error) across eight meadow patches within Maud Recreation Zone of Ningaloo Marine Park near Coral Bay, during summer season 2018. Values of 0 indicate neutrality, while positive and negative values indicate preference and avoidance, respectively
Foraging preferences of common invertivorous fishes for three microhabitat categories (canopy macroalgae, understory macroalgae and abiotic components) averaged (± standard error) across four meadow patches within Maud Recreation Zone of Ningaloo Marine Park near Coral Bay, during winter season 2018. Values of 0 indicate neutrality, while positive and negative values indicate preference and avoidance, respectively
Discussion
This study presents the seasonal foraging abundance and foraging microhabitat utilisation of invertivorous fish communities within tropical macroalgal meadows of Coral Bay, Ningaloo Marine Park, Western Australia. The family Labridae were the most abundant invertivorous taxa that foraged within the macroalgal meadows, making up over 70% of our foraging observations, followed by the families Mullidae and Lethrinidae. Our study revealed that fishes categorised as ‘invertivores’ have distinct foraging microhabitat preferences in canopy-forming macroalgal meadows and that individual species have different functional impacts within these systems. The invertivorous fish communities in this study can be divided into three categories of foraging specialists: ‘canopy forager’, ‘abiotic forager’ and ‘generalist’, based on their microhabitat preferences, highlighting a previously unappreciated aspect of functional complementarity within this particular trophic group. Surprisingly, almost all the invertivorous fish species avoided understory macroalgae while foraging, and this pattern was consistent between summer and winter, even though understory macroalgae are proportionally more available in winter.
Invertivorous fishes categorised as ‘canopy foragers’ in both summer and winter were from the family Labridae, whereas ‘generalists’ were from the family Lethrinidae in summer and a single labrid species in winter. The ‘abiotic foragers’ were from the families Labridae and Mullidae, with the mullids exclusively recorded under this category, suggesting that the division of the microhabitat niche axis is likely to be based on sharing similar morphological traits. For example, for labrids that are canopy foragers, their characteristically pointed snout and protruding canine teeth make it possible to flip the macroalgal blades to expose hidden epifaunal prey (Choat and Bellwood 1998; Froese and Pauly 2021), whereas labrids, which are considered to have good swimming abilities (higher fin aspect ratio to body size), are more capable of escaping from potential predators that use canopies as ambush sites (Fulton et al. 2001; Willis and Anderson 2003; Hoey and Bellwood 2011). However, the potential relationships between morphological traits and behavioural specialisation need to be further investigated, as morphology traits such as fin aspect ratio and body size, are not necessarily related to taxonomy.
Recent studies have compared epifaunal communities between neighbouring habitats across latitudes and seasons and found that epifaunal community structure can vary between habitats across microhabitat and seascapes, with strong seasonal fluctuations in their biomass driven by the availability of microhabitat (Chen et al. 2020, 2021; Fraser et al. 2020). In principle, this allows for assessment of whether differences in community composition between habitats translate either into differences in dietary target or nutritional quality. It is likely that particular dietary targets are more within communities at certain locations, driving specialisations in foraging microhabitat preferences of invertivorous fishes. In our study, we were unfortunately unable to collect specimens for gut content analysis to verify potential dietary targets that might drive the division of the foraging habitat resource axis. However, this represents a profitable future direction of research to test whether the prey selection and dietary targets of invertivorous fish species vary with microhabitat in macroalgal meadows.
Although the taxonomic composition of foraging invertivorous fishes in our survey varied seasonally, four of the five fish species which were observed foraging within the winter meadow patches showed consistent foraging microhabitat preferences in both seasons (Figs. 5, 6). This suggests that the observed microhabitat niche partitioning is likely to be based on real foraging specialisations, rather than just resulting from microhabitat crowding and resource competition in a given season. In our study, the foraging abundance of invertivorous fishes (in terms of individual or species) underwent dramatic declines in winter, associated with the extent of Sargassum canopy loss (Fig. 2, Tables 1, 2), indicating that canopy foragers may move to adjacent habitats due to the local absence of preferred microhabitats. Previous studies have shown the periodical migration of fish assemblages within marine macrophytal habitats which undergo systematic and predictable seasonal fluctuations (Green et al. 2009; Wilson et al. 2014; Lim et al. 2016). However, two canopy foraging labrid species ‘Cheilio inermis’ and ‘Coris caudimacula’ with large home range (72,000 m and 320 m, respectively, van Lier et al. 2018) continued to forage within the winter meadows, without moving to adjacent coral reefs, suggesting they are stronger habitat specialists than the other canopy foragers. Notably, one of the summer canopy foragers, Thalassoma lunare, shifted to being a generalist that utilised both canopy and understory macroalgae in winter, indicating that this species changed its foraging microhabitat preferences in response to fluctuations in the availability of favoured resources. However, this finding requires corroboration as only two of the four surveyed meadow patches in winter were included in our analysis (Supplemental Fig. S1h).
Surprisingly, only two abiotic foraging species ‘Halichoeres nebulosus’ and ‘Parupeneus spilurus’ were observed in sufficient numbers to measure their foraging preferences in the winter meadow patches despite there being no equivalent reduction in abiotic components compared with canopy macroalgae (so we did not expect to see a significant reduction in the foraging abundance of ‘abiotic foragers’ and ‘generalists’). This suggests that, for such non-canopy foraging species, macroalgal canopies may provide other important functions including nurseries for recruitment, or refuge from predators (Tano et al. 2017; Wilson et al. 2017). Once macroalgal canopies start to dieback, these satellite functions might be lost, meaning that generalists and abiotic foragers are forced to move to adjacent habitats.
Almost all the fish species documented in the current study showed a strong tendency to avoid foraging on understory macroalgae. This accords with previous studies of the foraging behaviour of individual fish species within macroalgal meadows (herbivorous Leptoscarus vaigiensis: Lim et al. 2016; invertivorous Xenojulis margaritaceus: Wenger et al. 2018). Potential factors that may discourage invertivores from foraging in the understory include: (1) nutritional differences and/or differences in taxonomic structure of epifaunal prey communities between the two microhabitats and (2) differential predation threat in the two microhabitats. Previous studies have suggested that canopy macroalgae are able to harbour a greater biomass of epifaunal invertebrates and/or to provide better quality of shelters than non-canopy species due to their more complex structure (Taylor and Cole 1994; Cacabelos et al. 2010; Carvalho et al. 2018; O'Brien et al. 2018). Hence, the understory macroalgae at Ningaloo Reef may represent a poorer dietary resource for invertivorous fishes. Interestingly, this pattern of avoidance of understory macroalgae was weaker in winter (Figs. 6, Supplemental Fig. S1), suggesting that understory macroalgae are a less undesirable habitat when canopy macroalgae are scarce.
Previous studies have already shown that environmental changes (either seasonal fluctuations or climate anomalies) can lead to the extensive loss of macroalgal canopy cover and can be replaced by less-complex algal species (Feng et al. 2013; Graba-Landry et al. 2020; Figueiredo et al. 2020; Chen et al. 2021). Given that future disturbance induced by climate change (e.g. extreme weather events, thermal anomalies) will become more frequent and more intense, a reduction in invertivorous fish abundance within canopy macroalgal meadows can be expected. This could dramatically reduce fishery production underpinned by the trophic links facilitated by invertivorous fishes, and an overall decline in trophic interactions by invertivorous fishes across all latitudes due to climate-driven thermal events has been predicted (Inagaki et al. 2020).
The refinement of foraging specialisations of invertivorous fishes based on foraging microhabitat preferences that we have presented here will aid future studies to identify the specific functional roles of invertivorous fishes and how these relate to ecosystem functioning, to yield a more conservative estimate of the level of functional redundancy within the ecosystem. This information will be important for management actions going forward. For example, over-exploitation of species that all fall within the ‘canopy forager’ role is likely to have consequences for top-down control of epifaunal invertebrate communities and cascading effects on primary producers. Moreover, as the foraging microhabitat preferences of invertivorous fish species are unravelled further, particular species may be found to play a unique role in facilitating particular trophic links between organisms. For example, previous studies of invertivorous fishes in the canopy macroalgal meadows of Ningaloo have tended to focus on fishery or recreational targets, especially fishes from the family Lethrinidae (Westera 2003; Wilson et al. 2010, 2014, 2017). However, due to the consistency of their abundance and foraging microhabitat preferences across seasons, canopy foragers such as the labrids are also likely to be vital components of macroalgal meadow ecosystems. As these ecosystems come under pressure from climate change (Smale and Wernberg 2013; Straub et al. 2019; Graba-Landry et al. 2020), the predicted range contractions of canopy macroalgal meadows will impact on associated invertivorous fish communities, especially on canopy specialists. Future research should examine the potential implications of loss of macroalgal meadow habitats for ecosystem dynamics, based on the refinements to functional specialisations of the species presented here.
References
Asher J, Williams ID, Harvey ES (2017) Mesophotic depth gradients impact reef fish assemblage composition and functional group partitioning in the main Hawaiian Islands. Front Mar Sci 4:98
Ashworth EC, Depczynski M, Holmes TH, Wilson SK (2014) Quantitative diet analysis of four mesopredators from a coral reef. J Fish Biol 84:1031–1045
Ba-Akdah MA, Satheesh S, Al-Sofyani AA (2016) Habitat preference and seasonal variability of epifaunal assemblages associated with macroalgal beds on the Central Red Sea coast, Saudi Arabia. J Mar Biol Assoc UK 96:1457–1467
Beck MW, Heck KL, Able KW, Childers DL, Eggleston DB, Gillanders BM, Halpern B, Hays CG, Hoshino K, Minello TJ, Orth RJ, Sheridan PF, Weinstein MR (2001) The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates. Bioscience 51:633–641
Bellwood DR, Choat JH (1990) A functional analysis of grazing in parrotfishes (family Scaridae): the ecological implications. In: Bruton MN (eds) Alternative life-history styles of fishes. Developments in environmental biology of fishes, vol 10. Springer, Dordrecht
Bellwood DR, Streit RP, Brandl SJ, Tebbett SB (2019) The meaning of the term ‘function’ in ecology: a coral reef perspective. Funct Ecol 33:948–961
Bergström L, Karlsson M, Bergström U, Pihl L, Kraufvelin P (2016) Distribution of mesopredatory fish determined by habitat variables in a predator-depleted coastal system. Mar Biol 163:201
Berkström C, Jones GP, McCormick MI, Srinivasan M (2012) Ecological versatility and its importance for the distribution and abundance of coral reef wrasses. Mar Ecol-Prog Ser 461:151–163
Brandl SJ, Bellwood DR (2014) Individual-based analyses reveal limited functional overlap in a coral reef fish community. J Anim Ecol 83:661–670
Brandl SJ, Robbins WD, Bellwood DR (2015) Exploring the nature of ecological specialization in a coral reef fish community: morphology, diet and foraging microhabitat use. Proc R Soc B 282:20151147
Brandl SJ, Casey JM, Meyer CP (2020) Dietary and habitat niche partitioning in congeneric cryptobenthic reef fish species. Coral Reefs 39:305–317
Cacabelos E, Olabarria C, Incera M, Troncoso JS (2010) Effects of habitat structure and tidal height on epifaunal assemblages associated with macroalgae. Estuar Coast Shelf Sci 89:43–52
Carvalho NF, Grande H, Rosa JS, Jacobucci GB (2018) The structure of gammarid amphipod (Crustacea, Peracarida) assemblages associated with Sargassum (Phaeophyta, Fucales) and their link with the structural complexity of algae. Hydrobiologia 820:245–254
Chaves LTC, Pereira PHC, Feitosa JLL (2013) Coral reef fish association with macroalgal beds on a tropical reef system in North-eastern Brazil. Mar Freshwater Res 64:1101–1111
Cheal AJ, MacNeil MA, Cripps E, Emslie MJ, Jonker M, Schaffelke B, Sweatman H (2010) Coral-macroalgal phase shifts or reef resilience: links with diversity and categor of herbivorous fishes on the Great Barrier Reef. Coral Reefs 29:1005–1015
Cheal A, Emslie M, Miller I, Sweatman H (2012) The distribution of herbivorous fishes on the Great Barrier Reef. Mar Biol 159:1143–1154
Chen YY, Cooper P, Fulton CJ (2020) Sargassum epifaunal communities vary with canopy size, predator biomass and seascape setting within a fringing coral reef ecosystem. Mar Ecol-Prog Ser 640:17–30
Chen YY, Cooper P, Fulton CJ, Fox RJ (2021) Quantifying epifaunal secondary production within tropical macroalgal meadows: Seasonality and sensitivity to canopy structure. Limnol Oceanogr 66:4267–4284
Choat H, Bellwood D (1998) Wrasses and Parrotfishes. In: Eschmeyer W, Paxton J (eds) Encyclopedia of fishes -, 2nd edn. Academic Press, San Diego, CA, pp 209–213
Choat J, Clements K, Robbins W (2002) The trophic status of herbivorous fishes on coral reefs. 1: dietary analyses. Mar Biol 140:613–623
Edgar GJ, Aoki M (1993) Resource limitation and fish predation: their importance to mobile epifauna associated with Japanese Sargassum. Oecologia 95:122–133
Feng M, McPhaden MJ, Xie SP, Hafner J (2013) La Niña forces unprecedented Leeuwin Current warming in 2011. Sci Rep 3:1277
Figueiredo CK, Duarte RC, Flores AA (2020) Ecosystem functioning of canopy-and turf-forming algae: contrasting supply of invertebrate prey to pelagic consumers. Mar Ecol-Prog Ser 647:79–92
Floeter SR, Krohling W, Gasparini JL, Ferreira CEL, Zalmon IR (2007) Reef fish community structure on coastal islands of the southeastern Brazil: the influence of exposure and benthic cover. Environ Biol of Fish 78:147–160
Fox RJ, Bellwood DR (2008) Remote video bioassays reveal the potential feeding impact of the rabbitfish Siganus canaliculatus (f : Siganidae) on an inner-shelf reef of the Great Barrier Reef. Coral Reefs 27:605–615
Fox RJ, Bellwood DR (2013) Niche partitioning of feeding microhabitats produces a unique function for herbivorous rabbitfishes (Perciformes, Siganidae) on coral reefs. Coral Reefs 32:13–23
Fraser KM, Stuart-Smith RD, Ling SD, Heather FJ, Edgar GJ (2020) Taxonomic composition of mobile epifaunal invertebrate assemblages on diverse benthic microhabitats from temperate to tropical reefs. Mar Ecol-Prog Ser 640:31–43
Froese R, Pauly D (2021) FishBase: World Wide Web electronic publication. http://www.fishbase.org, (02/2021)
Fulton C, Bellwood D, Wainwright P (2001) The relationship between swimming ability and habitat use in wrasses (Labridae). Mar Biol 139:25–33
Fulton CJ, Depczynski M, Holmes TH, Noble MM, Radford B, Wernberg T, Wilson SK (2014) Sea temperature shapes seasonal fluctuations in seaweed biomass within the Ningaloo coral reef ecosystem. Limnol Oceanogr 59:156–166
Fulton CJ, Noble MN, Radford B, Gallen C, Harasti D (2016) Microhabitat selectivity underpins regional indicators of fish abundance and replenishment. Ecol Indic 70:222–231
Fulton CJ, Abesamis RA, Berkström C, Depczynski M, Graham NAJ, Holmes TH, Kulbicki M, Noble MM, Radford BT, Tano S, Tinkler P, Wernberg T, Wilson SK (2019) Form and function of tropical macroalgal reefs in the Anthropocene. Funct Ecol 33:989–999
Fulton CJ, Berkström C, Wilson SK, Abesamis RA, Bradley M, Åkerlund C, Barrett LT, Bucol AA, Chacin DH, Chong-Seng KM, Coker DJ, Depczynski M, Eggertsen L, Eggertsen M, Ellis D, Evans RD, Graham NAJ, Hoey AS, Holmes TH, Kulbicki M, Leung PTY, Lam PKS, van Lier J, Matis PA, Noble MM, Pérez-Matus A, Piggott C, Radford BT, Tano S, Tinkler P (2020) Macroalgal meadow habitats support fish and fisheries in diverse tropical seascapes. Fish Fish 21:700–717
Graba-Landry AC, Loffler Z, McClure EC, Pratchett MS, Hoey AS (2020) Impaired growth and survival of tropical macroalgae (Sargassum spp.) at elevated temperatures. Coral Reefs 39:475–486
Graham NA, Bellwood DR, Cinner JE, Hughes TP, Norström AV, Nyström M (2013) Managing resilience to reverse phase shifts in coral reefs. Front Ecol Environ 11:541–548
Green AL, Bellwood DR (2009) Monitoring functional groups of herbivorous fishes as indicators of coral reef resilience - A practical guide for coral reef managers in the Asia Pacific region. IUCN working group on climate change and coral reefs. IUCN, Gland, Switzerland
Green BC, Smith DJ, Earley SE, Hepburn LJ, Underwood GJC (2009) Seasonal changes in community composition and trophic structure of fish populations of five salt marshes along the Essex coastline, United Kingdom. Estuar Coast Shelf S 85:247–256
Hoey AS, Bellwood DR (2009) Limited functional redundancy in a high diversity system: single species dominates key ecological process on coral reefs. Ecosystems 12:1316–1328
Hoey AS, Bellwood DR (2011) Suppression of herbivory by macroalgal density: a critical feedback on coral reefs? Ecol Lett 14:267–273
Hutchinson GE (1959) Homage to Santa Rosalia or why are there so many kinds of animals? Am Nat 93:145–159
Inagaki KY, Pennino MG, Floeter SR, Hay ME, Longo GO (2020) Trophic interactions will expand geographically but be less intense as oceans warm. Glob Change Biol 26:6805–6812
Kobryn HT, Wouters K, Beckley LE, Heege T (2013) Ningaloo Reef: shallow marine habitats mapped using a hyperspectral sensor. PLoS ONE 8:1–22
Krajewski JP, Floeter SR (2011) Reef fish community structure of the Fernando de Noronha Archipelago (Equatorial Western Atlantic): the influence of exposure and benthic composition. Environ Biol Fish 92:25
Kramer MJ, Bellwood O, Fulton CJ, Bellwood DR (2015) Refining the invertivore: diversity and specialisation in fish predation on coral reef crustaceans. Mar Biol 162:1779–1786
Kramer MJ, Bellwood O, Bellwood DR (2016) Foraging and microhabitat use by crustacean-feeding wrasses on coral reefs. Mar Ecol-Prog Ser 548:277–282
Kwak SN, Klumpp DW, Park JM (2015) Feeding relationships among juveniles of abundant fish species inhabiting tropical seagrass beds in Cockle Bay, North Queensland, Australia. N Z J Mar Freshw Res 49:205–223
Layton C, Fulton CJ (2014) Status-dependent foraging behaviour in coral reef wrasses. Coral Reefs 33:345–349
Lefevre CD, Bellwood DR (2010) Seasonality and dynamics in coral reef macroalgae: variation in condition and susceptibility to herbivory. Mar Biol 157:955–965
Leite FPP, Turra A (2003) Temporal variation in Sargassum biomass, Hypnea epiphytism and associated fauna. Braz Arch Biol Technol 46:665–671
Lewis LS, Anderson TW (2012) Top-down control of epifauna by fishes enhances seagrass production. Ecology 93:2746–2757
Lim IE, Wilson SK, Holmes TH, Noble MM, Fulton CJ (2016) Specialization within a shifting habitat mosaic underpins the seasonal abundance of a tropical fish. Ecosphere 7:1–13
Longo GO, Hay ME, Ferreira CEL, Floeter SR (2019) Trophic interactions across 61 degrees of latitude in the Western Atlantic. Glob Ecol Biogeogr 28:107–117
Lukoschek V, McCormick MI (2001) Ontogeny of diet changes in a tropical benthic carnivorous fish, Parupeneus barberinus (Mullidae): relationship between foraging behaviour, habitat use, jaw size, and prey selection. Mar Biol 138:1099–1113
MacArthur RH (1958) Population ecology of some warblers of northeastern coniferous forests. Ecology 39:599–619
Munday PL (2004) Habitat loss, resource specialization, and extinction on coral reefs. Glob Change Biol 10:1642–1647
Newcombe EM, Taylor RB (2010) Trophic cascade in a seaweed-epifauna-fish food chain. Mar Ecol-Prog Ser 408:161–167
O’Brien BS, Mello K, Litterer A, Dijkstra JA (2018) Seaweed structure shapes trophic interactions: a case study using a mid-trophic level fish species. J Exp Mar Biol Ecol 506:1–8
Parravicini V, Casey JM, Schiettekatte NMD, Brandl SJ, Pozas-Schacre C, Carlot J, Edgar GJ, Graham NAJ, Harmelin-Vivien M, Kulbicki M, Strona G, Stuart-Smith RD (2020) Delineating reef fish trophic guilds with global gut content data synthesis and phylogeny. PLoS Biol 18:e3000702
Pratchett MS, Thompson CA, Hoey AS, Cowman PF, Wilson SK (2018) Effects of coral bleaching and coral loss on the structure and function of reef fish assemblages. In: van Oppen MJH, Lough J (eds) Coral bleaching: patterns, processes, causes and consequences. Springer, Basel, Switzerland, pp 265–293
Randall JE, Allen GR, Steene RC (1997) Fishes of the Great Barrier Reef and Coral Sea. University of Hawaii Press, Hawaii, Honolulu
Rasher DB, Hoey AS, Hay ME (2013) Consumer diversity interacts with prey defenses to drive ecosystem function. Ecology 94:1347–1358
Sambrook K, Hoey AS, Andréfouët S, Cumming GS, Duce S, Bonin MC (2019) Beyond the reef: the widespread use of non-reef habitats by coral reef fishes. Fish Fish 20:903–920
Smale DA, Wernberg T (2013) Extreme climatic event drives range contraction of a habitat-forming species. Proc R Soc B-Biol Sci 280:20122829
Straub SC, Wernberg T, Thomsen MS, Moore PJ, Burrows MT, Harvey B, Smale DA (2019) Resistance, extinction, and everything in between - the diverse responses of seaweeds to marine heatwaves. Front Mar Sci 6:763
Streit RP, Hoey AS, Bellwood DR (2015) Feeding characteristics reveal functional distinctions among browsing herbivorous fishes on coral reefs. Coral Reefs 34:1037–1047
Sumner NR, Williamson PC, Malseed BE (2002) A 12-month survey of recreational fishing in the Gascoyne bioregion of Western Australia during 1998–99. Western Australia: fisheries Research Report No. 139, Department of Fisheries
Tano S, Eggertsen M, Wikström SA, Berkström C, Buriyo AS, Hailing C (2016) Tropical seaweed beds are important habitats for mobile invertebrate epifauna. Estuar Coast Shelf Sci 183:1–12
Tano SA, Eggertsen M, Wikström SA, Berkström C, Buriyo AS, Halling C (2017) Tropical seaweed beds as important habitats for juvenile fish. Mar Freshwater Res 68:1921–1934
Taylor RB (1998) Seasonal variation in assemblages of mobile epifauna inhabiting three subtidal brown seaweeds in northeastern New Zealand. Hydrobiologia 361:25–35
Taylor RB, Cole RG (1994) Mobile epifauna on subtidal brown seaweeds in northeastern New Zealand. Mar Ecol-Prog Ser 115:271–282
Thrush SF, Dayton PK (2010) What can ecology contribute to ecosystem-based management? Annu Rev Mar Sci 2:419–441
van Lier JR, Wilson SK, Depczynski M, Wenger LN, Fulton CJ (2018) Habitat connectivity and complexity underpin fish community structure across a seascape of tropical macroalgae meadows. Landsc Ecol 33:1287–1300
Vanderploeg HA, Scavia D (1979) Calculation and use of selectivity coefficients of feeding - zooplankton grazing. Ecol Model 7:135–149
Villéger S, Brosse S, Mouchet M, Mouillot D, Vanni MJ (2017) Functional ecology of fish: current approaches and future challenges. Aquat Sci 79:783–801
Wenger LN, van Lier JR, Fulton CJ (2018) Microhabitat selectivity shapes the seascape ecology of a carnivorous macroalgae-associated tropical fish. Mar Ecol-Prog Ser 590:187–200
Westera MB (2003) The effect of recreational fishing on targeted fishes and trophic structure, in a coral reef marine park. https://ro.ecu.edu.au/theses/1499
Westmacott S, Teleki K, Wells S, West JM (2000) Management of bleached and severely damaged coral reefs. International Union for the Conservation of Nature, Gland, Switzerland.
Willis TJ, Anderson MJ (2003) Structure of cryptic reef fish assemblages: relationships with habitat characteristics and predator density. Mar Ecol-Prog Ser 257:209–221
Wilson SK, Fisher R, Pratchett MS, Graham NAJ, Dulvy NK, Turner RA, Cakacaka A, Polunin NVC, Rushton SP (2008a) Exploitation and habitat degradation as agents of change within coral reef fish communities. Glob Change Biol 14:2796–2809
Wilson SK, Burgess SC, Cheal AJ, Emslie M, Fisher R, Miller I, Polunin NVC, Sweatman HPA (2008b) Habitat utilization by coral reef fish: implications for specialists vs. generalists in a changing environment. J Anim Ecol 77:220–228
Wilson SK, Depczynski M, Fisher R, Holmes TH, O’Leary RA, Tinkler P (2010) Habitat associations of juvenile fish at Ningaloo Reef, Western Australia: the importance of coral and algae. PLoS ONE 5:e15185
Wilson SK, Fulton CJ, Depczynski M, Holmes TH, Noble MM, Radford B, Tinkler P (2014) Seasonal changes in habitat structure underpin shifts in macroalgae-associated tropical fish communities. Mar Biol 161:2597–2607
Wilson SK, Depczynski M, Holmes TH, Noble MM, Radford BT, Tinkler P, Fulton CJ (2017) Climatic conditions and nursery habitat quality provide indicators of reef fish recruitment strength. Limnol Oceanogr 62:1868–1880
Wong CL, Phang SM (2004) Biomass production of two Sargassum species at Cape Rachado, Malaysia. Hydrobiologia 512:79–88
Acknowledgements
We thank M. Eggertsen, D. Ellis, C. Fulton and J. van Lier for assistance in the field. Funding for this study was provided by the Australian National University (Research School of Biology), the Taiwanese Ministry of Education (Postgraduate research funding to Y-Y.C) and the Australian Coral Reef Society (Student Grant Award to Y-Y.C).
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Topic Editor Andrew Hoey
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, YY., Jennions, M. & Fox, R.J. Foraging microhabitat preferences of invertivorous fishes within tropical macroalgal meadows: identification of canopy specialists. Coral Reefs 41, 1511–1522 (2022). https://doi.org/10.1007/s00338-022-02298-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-022-02298-9