Abstract
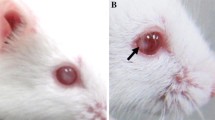
To date around 140 genetic alleles have been identified as being responsible for mouse cataract pathology, including Crya, Cryb, Cryg, Maf, Pax6, Pitx3, Sox, Connexins, MIP, and Lim-2. We obtained a dominant cataract mouse model from a spontaneous mutation in the F1 hybrids of outbred strain ICR mice crossed to the inbred strain BALB/cJ mice. Heterozygous and homozygous mutants expressed a nuclear cataract in both eyes. In 8-day-old mice, histological analysis showed that polygon epithelial cells were in the equatorial region and cortex underneath, and vacuole and sponge-like degeneration were in the cortical area underneath the posterior lens capsule. The nucleus of the lens was a deeply stained pink, with the shorter fibers losing their normal arrangement. For the entire eye, there was a blank zone in the equatorial region in 8-day-old mice; however, there was a certain degree of atrophy in cornea tension and retina in the lens in 3-month-old mice. The lens had been serious damaged in the homozygous mutants. For mutation mapping, heterozygous carriers were mated to wild-type C3H/HeJ mice, and offspring (F1 generation) with cataracts were backcrossed to the wild-type C3H/HeJ mice again. N2 mice with cataracts were used for genotyping. Using genome-wide linkage analysis, the mutation was mapped to chromosome 1 and the Cryg gene cluster between two markers was confirmed as the candidate gene. After direct sequencing the cDNA of the Cryg gene cluster, a 1-bp deletion was found in exon 3 of the Crygc gene, leading to a stop codon at the 76th amino acid of exon 3 which results in production of a truncated protein in mutant mice (Leu160Stop). Bioinformatic analysis of the mutant γC-crystallin reveals that the COOH-terminal of the mutant protein deletes a β-sheet, which affects the function of the lens proteins and leads to the development of cataracts.









Similar content being viewed by others
References
Arnold K, Bordoli L, Kopp J, Schwede T (2006) The SWISS-MODEL Workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22:195–201
Brown SD, Balling R (2001) Systematic approaches to mouse mutagenesis. Curr Opin Genet Dev 11:268–273
Bu L, Yan S, Jin M, Jin Y, Yu C (2002) The gamma S-crystallin gene is mutated in autosomal recessive cataract in mouse. Genomics 80:38–44
Gonzalez-Huerta LM, Messina-Baas OM, Cuevas-Covarrubias SA (2007) A family with autosomal dominant primary congenital cataract associated with a CRYGC mutation: evidence of clinical heterogeneity. Mol Vis 13:1333–1338
Graw J (1997) The crystallins: genes, proteins, and diseases. Biol Chem 378:1331–1348
Graw J (2004) Congenital hereditary cataracts. Int J Dev Biol 48:1031–1044
Graw J (2009) Mouse models of cataract. J Genet 88:469–486
Graw J, Löster J (2003) Developmental genetics in ophthalmology. Ophthalmic Genet 24:1–33
Graw J, Klopp N, Löster J, Soewarto D, Fuchs H et al (2001) ENU-induced mutation in mice leads to the expression of a novel protein in the eye and to dominant cataracts. Genetics 157:1313–1320
Graw J, Neubäuser-Klaus A, Löster J, Favor J (2002) A 6-bp deletion in the Cryg gene leading to a nuclear and radial cataract in the mouse. Invest Ophthalmol Vis Sci 43:236–240
Graw J, Neubäuser-Klaus A, Klopp N, Selby PB, Löster J et al (2004) Genetic and allelic heterogeneity of Cryg mutations in eight distinct forms of dominant cataract in the mouse. Invest Ophthalmol Vis Sci 45:1202–1213
Guex N, Peitsch MC (1997) SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modelling. Electrophoresis 18:2714–2723
Héon E, Priston M, Schorderet DF, Billingsley GD, Girard PO et al (1999) The γ-crystallins and human cataracts: a puzzle made clearer. Am J Hum Genet 65:1261–1267
Ji Y, Lu Y, Kong X, Yan S (2007) The anatomical and pathological changes in the crystalline lens of a congenital γS-crystallin gene mutated mouse model. Chin J Optom Ophthalmol 9:145–148
Kang M, Cho JW, Kim JK, Kim E, Kim JY et al (2008) Fine localization of a new cataract locus, Kec, on mouse chromosome 14 and exclusion of candidate genes as the gene that causes cataract in the Kec mouse. BMP Rep 41:651–656
Klopp N, Favor J, Löster J, Lutz RB, Neuhäuser-Klaus A et al (1998) Three murine cataract mutants (Cat2) are defective in different γ-crystallin genes. Genomics 52:152–158
Kratochvilova J, Favor J (1992) Allelism tests of 15 dominant cataract mutations in mice. Genet Res 59:199–203
Li L, Chang B, Cheng C, Chang D, Hawes NL et al (2008) Dense nuclear cataract caused by the gamma B-crystallin S11R point mutation. Invest Ophthalmol Vis Sci 49:304–309
Liu Y, Zhang X, Luo L, Wu M, Zeng R et al (2006) A novel alphaB-crystallin mutation associated with autosomal dominant congenital lamellar cataract. Invest Ophthalmol Vis Sci 47:1069–1075
Matteson PG, Desai J, Korstanje R, Lazar G, Borsuk TE et al (2008) The orphan G protein-coupled recptor, Gpr161, encodes the vacuolated lens locus and controls neurulation and lens development. Physiol Genomics 105:2088–2093
Omi N, Kiyokawa E, Matsuda M, Kinoshita K, Yamada S et al (2008) Mutation of Dock5, a member of the guanine exchange factor Dock180 superfamily, in the rupture of lens cataract mouse. Exp Eye Res 86:828–834
Ren Z, Li A, Shastry BS, Padma T, Ayyagari R et al (2000) A 5-base insertion in the γC-crystallin gene is associated with autosomal dominant variable zonular pulverulent cataract. Hum Genet 106:531–537
Sandilands A, Hutcheson AM, Long HA, Prescott AR, Vrensen G et al (2002) Altered aggregation 105: properties of mutant γ-crystallins cause inherited cataract. EMBO J 21:6005–6014
Sandilands A, Wang X, Hutcheson AM, James J, Prescott AR et al (2004) Bfsp2 mutation found in mouse 129 strains causes the loss of CP49 and induces vimentin-dependent changes in the lens fibre cell cytoskeleton. Exp Eye Res 78:109–123
Santana A, Waiswol M, Arcieri ES, Cabral de Vasconcellos JP, Barbosa de Melo M (2009) Mutation analysis of CRYAA, CRYGC, and CRYGD associated with autosomal dominant congenital cataract in Brazilian families. Mol Vis 15:793–800
Santhiya ST, Manohar MS, Rawlley D, Vijayalakshmi P, Namperumalsamy P et al (2002) Novel mutations in the γ-crystallin genes cause autosomal dominant congenital cataracts. J Med Genet 39:352–358
Schwede T, Kopp J, Guex N, Peitsch MC (2003) SWISS-MODEL: an automated protein homology-modeling server. Nucleic Acids Res 31:3381–3385
Shi X, Cui B, Wang Z, Weng L, Xu Z et al (2009) Removal of Hsf4 leads to cataract development in mice through down-regulation of gammaS-crystallin and Bfsp expression. BMC Mol Biol 10:10
Slingsby C, Clout NJ (1999) Structure of the crystallins. Eye 13:395–402
Talamas E, Jackson L, Koeberl M, Jackson T, McElwee JL et al (2006) Early transposable element insertion in intron 9 of the Hsf4 gene results in autosomal recessive cataracts in lop11 and ldisl mice. Genomics 88:44–51
Wistow GJ, Piatigorsky J (1988) Lens crystallins: the evolution and expression of proteins for a highly specialized tissue. Annu Rev Biochem 57:479–504
Yao K, Jin C, Zhu N, Wang W, Wu R et al (2008) A nonsense mutation in CRYGC associated with autosomal dominant congenital nuclear cataract in a Chinese family. Mol Vis 14:1272–1276
Zhao G, Yang Y, Zhang R, Gu J, Xu P et al (2009) Cultivation of a mouse model of inherited cataract (BALB/c-Cat)―A preliminary report. Chin J Comput Med 19:63–65
Zhao L, Bao S, Zhao G, Liang Y, Li K et al (2010) Mutation, distribution, positional cloning for cataract in house mouse. Chin J Comput Med 20:62–66
Acknowledgments
The authors are grateful to the Eye Ear Nose and Throat Hospital affiliated with Fudan University for histological analysis. This work was supported by grants from Key Programs of the Science and Technology Commission Foundation of Shanghai (No. 09140901100) and National Natural Science Foundation of China (30700529).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhao, L., Li, K., Bao, S. et al. A 1-bp deletion in the γC-crystallin leads to dominant cataracts in mice. Mamm Genome 21, 361–369 (2010). https://doi.org/10.1007/s00335-010-9275-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00335-010-9275-5