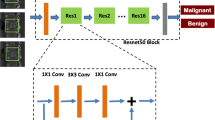
Abstract
Objective
To test the diagnostic performance of a deep-learning Two-Stream Compare and Contrast Network (TSCCN) model for differentiating benign and malignant vertebral compression fractures (VCFs) based on MRI.
Methods
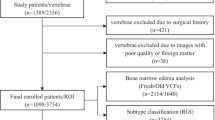
We tested a deep-learning system in 123 benign and 86 malignant VCFs. The median sagittal T1-weighted images (T1WI), T2-weighted images with fat suppression (T2WI-FS), and a combination of both (thereafter, T1WI/T2WI-FS) were used to validate TSCCN. The receiver operator characteristic (ROC) curve was analyzed to evaluate the performance of TSCCN. The accuracy, sensitivity, and specificity of TSCCN in differentiating benign and malignant VCFs were calculated and compared with radiologists’ assessments. Intraclass correlation coefficients (ICCs) were tested to find intra- and inter-observer agreement of radiologists in differentiating malignant from benign VCFs.
Results
The AUC of the ROC plots of TSCCN according to T1WI, T2WI-FS, and T1WI/T2WI-FS images were 99.2%, 91.7%, and 98.2%, respectively. The accuracy of T1W, T2WI-FS, and T1W/T2WI-FS based on TSCCN was 95.2%, 90.4%, and 96.2%, respectively, greater than that achieved by radiologists. Further, the specificity of T1W, T2WI-FS, and T1W/T2WI-FS based on TSCCN was higher at 98.4%, 94.3%, and 99.2% than that achieved by radiologists. The intra- and inter-observer agreements of radiologists were 0.79–0.85 and 0.79–0.80 for T1WI, 0.65–0.72 and 0.70–0.74 for T2WI-FS, and 0.83–0.88 and 0.83–0.84 for T1WI/T2WI-FS.
Conclusion
The TSCCN model showed better diagnostic performance than radiologists for automatically identifying benign or malignant VCFs, and is a potentially helpful tool for future clinical application.
Clinical relevance statement
TSCCN-assisted MRI has shown superior performance in distinguishing benign and malignant vertebral compression fractures compared to radiologists. This technology has the value to enhance diagnostic accuracy, sensitivity, and specificity. Further integration into clinical practice is required to optimize patient management.
Key Points
• The Two-Stream Compare and Contrast Network (TSCCN) model showed better diagnostic performance than radiologists for identifying benign vs malignant vertebral compression fractures.
• The processing of TSCCN is fast and stable, better than the subjective evaluation by radiologists in diagnosing vertebral compression fractures.
• The TSCCN model provides options for developing a fully automated, streamlined artificial intelligence diagnostic tool.




Similar content being viewed by others
Abbreviations
- PACS:
-
Picture archiving and communication system
- STIR:
-
Short-TI inversion recovery
- T1WI:
-
T1-weighted image
- T2WI-FS:
-
T2-weighted image fat suppression
- TSCCN:
-
Two-Stream Compare and Contrast Network
- VCF:
-
Vertebral compression fracture
References
Goldstein CL, Chutkan NB, Choma TJ, Orr RD (2015) Management of the elderly with vertebral compression fractures. Neurosurgery 77:S33–S45. https://doi.org/10.1227/neu.0000000000000947
Kim DH, Vaccaro AR (2006) Osteoporotic compression fractures of the spine; current options and considerations for treatment. Spine J 6:479–487. https://doi.org/10.1016/j.spinee.2006.04.013
Montagu A, Speirs A, Baldock J et al (2012) A review of vertebroplasty for osteoporotic and malignant vertebral compression fractures. Age Ageing 41:450–455. https://doi.org/10.1093/ageing/afs024
Coleman RE (2006) Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res 12:6243s–6249s. https://doi.org/10.1158/1078-0432.ccr-06-0931
Thawait SK, Marcus MA, Morrison WB, Klufas RA, Eng J, Carrino JA (2012) Research synthesis. Spine (Phila Pa 1976) 37:E736–E744. https://doi.org/10.1097/brs.0b013e3182458cac
Azevedo-Marques PM, Spagnoli HF, Frighetto-Pereira L, et al (2015) Classification of vertebral compression fractures in magnetic resonance images using spectral and fractal analysis. 2015 37th Annu Int Conf Ieee Eng Medicine Biology Soc Embc 2015:723–726. https://doi.org/10.1109/embc.2015.7318464
Uetani M, Hashmi R, Hayashi K (2004) Malignant and benign compression fractures: differentiation and diagnostic pitfalls on MRI. Clin Radiol 59:124–131. https://doi.org/10.1016/j.crad.2003.07.005
Chen W, Liu X, Li K et al (2022) A deep-learning model for identifying fresh vertebral compression fractures on digital radiography. Eur Radiol 32:1496–1505. https://doi.org/10.1007/s00330-021-08247-4
Yasaka K, Tanishima T, Ohtake Y et al (2022) Deep learning reconstruction for 1.5 T cervical spine MRI: effect on interobserver agreement in the evaluation of degenerative changes. Eur Radiol 32:6118–6125. https://doi.org/10.1007/s00330-022-08729-z
Roberts MG, Pacheco EMB, Mohankumar R, Cootes TF, Adams JE (2010) Detection of vertebral fractures in DXA VFA images using statistical models of appearance and a semi-automatic segmentation. Osteoporosis Int 21:2037–2046. https://doi.org/10.1007/s00198-009-1169-6
Burns JE, Yao J, Summers RM (2017) Vertebral body compression fractures and bone density: automated detection and classification on CT images. Radiology 284:788–797. https://doi.org/10.1148/radiol.2017162100
Frighetto-Pereira L, Rangayyan RM, Metzner, de Azevedo-Marques PM, Nogueira-Barbosa MH (2016) Shape, texture and statistical features for classification of benign and malignant vertebral compression fractures in magnetic resonance images. Comput Biol Med 73:147–156. https://doi.org/10.1016/j.compbiomed.2016.04.006
Tomita N, Cheung YY, Hassanpour S (2018) Deep neural networks for automatic detection of osteoporotic vertebral fractures on CT scans. Comput Biol Med 98:8–15. https://doi.org/10.1016/j.compbiomed.2018.05.011
Frighetto-Pereira L, Menezes-Reis R, Metzner GA, Rangayyan RM, Azevedo-Marques PM, Nogueira-Barbosa MH (2015) Semiautomatic classification of benign versus malignant vertebral compression fractures using texture and gray-level features in magnetic resonance images. 2015 Ieee 28th Int Symposium Comput Medical Syst 88–92. https://doi.org/10.1109/cbms.2015.37
Feng S, Liu B, Zhang Y, Zhang X, Li Y (2021) Two-stream compare and contrast network for vertebral compression fracture diagnosis. IEEE Trans Med Imaging 40:2496–2506. https://doi.org/10.1109/tmi.2021.3080991
Chhapola V, Kanwal SK, Brar R (2014) Reporting standards for Bland-Altman agreement analysis in laboratory research: a cross-sectional survey of current practice. Ann Clin Biochem 52:382–386. https://doi.org/10.1177/0004563214553438
Koo TK, Li MY (2016) A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Medicine 15:155–163. https://doi.org/10.1016/j.jcm.2016.02.012
Yoda T, Maki S, Furuya T et al (2022) Automated differentiation between osteoporotic vertebral fracture and malignant vertebral fracture on MRI using a deep convolutional neural network. Spine (Phila Pa 1976) 47:E347–E352. https://doi.org/10.1097/brs.0000000000004307
Varoquaux G, Cheplygina V (2022) Machine learning for medical imaging: methodological failures and recommendations for the future. NPJ Digital Med 5:48. https://doi.org/10.1038/s41746-022-00592-y
Castiglioni I, Rundo L, Codari M et al (2021) AI applications to medical images: from machine learning to deep learning. Phys Med 83:9–24. https://doi.org/10.1016/j.ejmp.2021.02.006
Mazurowski MA, Buda M, Saha A, Bashir MR (2019) Deep learning in radiology: an overview of the concepts and a survey of the state of the art with focus on MRI. J Magn Reson Imaging 49:939–954. https://doi.org/10.1002/jmri.26534
LeCun Y, Kavukcuoglu K, Farabet C (2010) Convolutional networks and applications in vision. Ieee Int Symposium Circuits Syst Iscas 2010:253–256. https://doi.org/10.1109/iscas.2010.5537907
Moffit B, Reicher M, Lufkin R, Bentson J (1988) Comparison of T1 and T2 weighted images of the lumbar spine. Comput Med Imag Grap 12:271–276. https://doi.org/10.1016/0895-6111(88)90037-7
Romeo V, Ugga L, Stanzione A, Cocozza S, Cuocolo R, Brunetti A (2019) Differential diagnosis of benign and malignant vertebral compression fractures using conventional and advanced MRI techniques. BJR Open 1:20180033. https://doi.org/10.1259/bjro.20180033
Funding
This study has received funding by the National Natural Science Foundation of China [81671673, 81871329]; the Shanghai Municipal Education Commission-Gaofeng Clinical Medicine Grant Support [2016427]; Shanghai Key Clinical Specialty (No. shslczdzk03203); the Shanghai Key Discipline of Medical Imaging [2017ZZ02005]; and the Foundation of Shanghai Sixth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine [X-2298].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Yuehua Li.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise. No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
No.
Methodology
• retrospective
• diagnostic study
• performed at one institution
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, B., Jin, Y., Feng, S. et al. Benign vs malignant vertebral compression fractures with MRI: a comparison between automatic deep learning network and radiologist’s assessment. Eur Radiol 33, 5060–5068 (2023). https://doi.org/10.1007/s00330-023-09713-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-09713-x