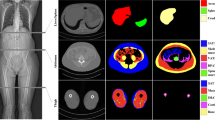
Abstract
Objectives
To compare volumetric CT with DL-based fully automated segmentation and dual-energy X-ray absorptiometry (DXA) in the measurement of thigh tissue composition.
Methods
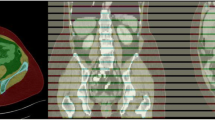
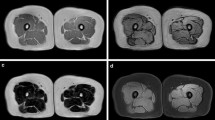
This prospective study was performed from January 2019 to December 2020. The participants underwent DXA to determine the body composition of the whole body and thigh. CT was performed in the thigh region; the images were automatically segmented into three muscle groups and adipose tissue by custom-developed DL-based automated segmentation software. Subsequently, the program reported the tissue composition of the thigh. The correlation and agreement between variables measured by DXA and CT were assessed. Then, CT thigh tissue volume prediction equations based on DXA-derived thigh tissue mass were developed using a general linear model.
Results
In total, 100 patients (mean age, 44.9 years; 60 women) were evaluated. There was a strong correlation between the CT and DXA measurements (R = 0.813~0.98, p < 0.001). There was no significant difference in total soft tissue mass between DXA and CT measurement (p = 0.183). However, DXA overestimated thigh lean (muscle) mass and underestimated thigh total fat mass (p < 0.001). The DXA-derived lean mass was an average of 10% higher than the CT-derived lean mass and 47% higher than the CT-derived lean muscle mass. The DXA-derived total fat mass was approximately 20% lower than the CT-derived total fat mass. The predicted CT tissue volume using DXA-derived data was highly correlated with actual CT-measured tissue volume in the validation group (R2 = 0.96~0.97, p < 0.001).
Conclusions
Volumetric CT measurements with DL-based fully automated segmentation are a rapid and more accurate method for measuring thigh tissue composition.
Key Points
• There was a positive correlation between CT and DXA measurements in both the whole body and thigh.
• DXA overestimated thigh lean mass by 10%, lean muscle mass by 47%, but underestimated total fat mass by 20% compared to the CT method.
• The equations for predicting CT volume (cm 3 ) were developed using DXA data (g), age, height (cm), and body weight (kg) and good model performance was proven in the validation study.






Similar content being viewed by others
Abbreviations
- DL:
-
Deep learning
- DXA:
-
Dual-energy X-ray absorptiometry
References
Wolfe RR (2006) The underappreciated role of muscle in health and disease. Am J Clin Nutr 84:475–482
Tavoian D, Ampomah K, Amano S, Law TD, Clark BC (2019) Changes in DXA-derived lean mass and MRI-derived cross-sectional area of the thigh are modestly associated. Sci Rep 9:10028
Lee K, Shin Y, Huh J et al (2019) Recent issues on body composition imaging for sarcopenia evaluation. Korean J Radiol 20:205–217
Boutin RD, Yao L, Canter RJ, Lenchik L (2015) Sarcopenia: current concepts and imaging implications. AJR Am J Roentgenol 205:W255–W266
Ruhdorfer A, Wirth W, Eckstein F (2015) Relationship between isometric thigh muscle strength and minimum clinically important differences in knee function in osteoarthritis: data from the osteoarthritis initiative. Arthritis Care Res 67:509–518
Ruhdorfer A, Wirth W, Eckstein F (2017) Association of knee pain with a reduction in thigh muscle strength – a cross-sectional analysis including 4553 osteoarthritis initiative participants. Osteoarthritis Cartilage 25:658–666
Segal NA, Torner JC, Felson D et al (2009) Effect of thigh strength on incident radiographic and symptomatic knee osteoarthritis in a longitudinal cohort. Arthritis Rheum 61:1210–1217
Segal NA, Glass NA, Torner J et al (2010) Quadriceps weakness predicts risk for knee joint space narrowing in women in the MOST cohort. Osteoarthritis Cartilage 18:769–775
Øiestad BE, Juhl CB, Eitzen I, Thorlund JB (2015) Knee extensor muscle weakness is a risk factor for development of knee osteoarthritis. A systematic review and meta-analysis. Osteoarthritis Cartilage 23:171–177
Culvenor AG, Wirth W, Ruhdorfer A, Eckstein F (2016) Thigh muscle strength predicts knee replacement risk independent of radiographic disease and pain in women: data from the osteoarthritis initiative. Arthritis Rheumatol 68:1145-1155
Levine JA, Abboud L, Barry M, Reed JE, Sheedy PF (1985) Jensen MD (2000) Measuring leg muscle and fat mass in humans: comparison of CT and dual-energy X-ray absorptiometry. J Appl Physiol (1985) 88:452–456
Engelke K, Museyko O, Wang L, Laredo JD (2018) Quantitative analysis of skeletal muscle by computed tomography imaging-State of the art. J Orthop Translat 15:91–103
Kim TN, Park MS, Lee EJ et al (2017) Comparisons of three different methods for defining sarcopenia: an aspect of cardiometabolic risk. Sci Rep 7:6491
Bredella MA, Ghomi RH, Thomas BJ et al (2010) Comparison of DXA and CT in the assessment of body composition in premenopausal women with obesity and anorexia nervosa. Obesity (Silver Spring) 18:2227–2233
Visser M, Kritchevsky SB, Goodpaster BH et al (2002) Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc 50:897–904
Davidson FE, Matsha TE, Erasmus RT, Ismail S, Kengne AP, Goedecke JH (2020) Comparison of single-slice CT and DXA-derived measures of central adiposity in South African women. Eur J Clin Nutr 74:1282–1289
Hansen RD, Williamson DA, Finnegan TP et al (2007) Estimation of thigh muscle cross-sectional area by dual-energy X-ray absorptiometry in frail elderly patients. Am J Clin Nutr 86:952–958
Wang W, Wang Z, Faith MS, Kotler D, Shih R (1985) Heymsfield SB (1999) Regional skeletal muscle measurement: evaluation of new dual-energy X-ray absorptiometry model. J Appl Physiol (1985) 87:1163–1171
Wang ZM, Visser M, Ma R et al (1985) (1996) Skeletal muscle mass: evaluation of neutron activation and dual-energy X-ray absorptiometry methods. J Appl Physiol (1985) 80:824–831
Ding J, Cao P, Chang HC, Gao Y, Chan SHS, Vardhanabhuti V (2020) Deep learning-based thigh muscle segmentation for reproducible fat fraction quantification using fat-water decomposition MRI. Insights Imaging 11:128
Hiasa Y, Otake Y, Takao M, Ogawa T, Sugano N, Sato Y (2020) Automated muscle segmentation from clinical CT using Bayesian U-Net for personalized musculoskeletal modeling. IEEE Trans Med Imaging 39:1030–1040
Heymsfield SB, Smith R, Aulet M et al (1990) Appendicular skeletal muscle mass: measurement by dual-photon absorptiometry. Am J Clin Nutr 52:214–218
Aubrey J, Esfandiari N, Baracos VE et al (2014) Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol (Oxf) 210:489–497
Yoshizumi T, Nakamura T, Yamane M et al (1999) Abdominal fat: standardized technique for measurement at CT. Radiology 211:283–286
Visser M, Fuerst T, Lang T, Salamone L, Harris TB (1985) (1999) Validity of fan-beam dual-energy X-ray absorptiometry for measuring fat-free mass and leg muscle mass. Health, Aging, and Body Composition Study--Dual-Energy X-ray Absorptiometry and Body Composition Working Group. J Appl Physiol (1985) 87:1513–1520
Chowdhury B, Sjostrom L, Alpsten M, Kostanty J, Kvist H, Lofgren R (1994) A multicompartment body composition technique based on computerized tomography. Int J Obes Relat Metab Disord 18:219–234
(1979) Report of the task group on reference man. Ann ICRP 3:iii. 10.1016/0146-6453(79)90123-4
Kim J, Shen W, Gallagher D et al (2006) Total-body skeletal muscle mass: estimation by dual-energy X-ray absorptiometry in children and adolescents. Am J Clin Nutr 84:1014–1020
Bland JM, Altman DG (1999) Measuring agreement in method comparison studies. Stat Methods Med Res 8:135–160
Maden-Wilkinson TM, Degens H, Jones DA, McPhee JS (2013) Comparison of MRI and DXA to measure muscle size and age-related atrophy in thigh muscles. J Musculoskelet Neuronal Interact 13:320–328
Euser AM, Dekker FW, le Cessie S (2008) A practical approach to Bland-Altman plots and variation coefficients for log transformed variables. J Clin Epidemiol 61:978–982
Brady SL, Trout AT, Somasundaram E, Anton CG, Li Y, Dillman JR (2021) Improving image quality and reducing radiation dose for pediatric CT by using deep learning reconstruction. Radiology 298:180–188
Mayo-Smith WW, Hara AK, Mahesh M, Sahani DV, Pavlicek W (2014) How I do it: managing radiation dose in CT. Radiology 273:657–672
Kemnitz J, Baumgartner CF, Eckstein F et al (2020) Clinical evaluation of fully automated thigh muscle and adipose tissue segmentation using a U-Net deep learning architecture in context of osteoarthritic knee pain. MAGMA 33:483–493
Funding
This work was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MSIT) (NRF-2017M3A9D8064198).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Ja-Young Choi M.D., Ph.D.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• prospective
• pobservational
• pperformed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yoo, H.J., Kim, Y.J., Hong, H. et al. Deep learning–based fully automated body composition analysis of thigh CT: comparison with DXA measurement. Eur Radiol 32, 7601–7611 (2022). https://doi.org/10.1007/s00330-022-08770-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-08770-y