Abstract
Objectives
To develop and validate a machine learning based automated segmentation method that jointly analyzes the four contrasts provided by Dixon MRI technique for improved thigh composition segmentation accuracy.
Materials and methods
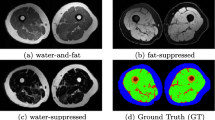
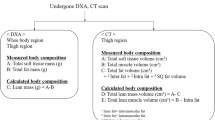
The automatic detection of body composition is formulized as a three-class classification issue. Each image voxel in the training dataset is assigned with a correct label. A voxel classifier is trained and subsequently used to predict unseen data. Morphological operations are finally applied to generate volumetric segmented images for different structures. We applied this algorithm on datasets of (1) four contrast images, (2) water and fat images, and (3) unsuppressed images acquired from 190 subjects.
Results
The proposed method using four contrasts achieved most accurate and robust segmentation compared to the use of combined fat and water images and the use of unsuppressed image, average Dice coefficients of 0.94 ± 0.03, 0.96 ± 0.03, 0.80 ± 0.03, and 0.97 ± 0.01 has been achieved to bone region, subcutaneous adipose tissue (SAT), inter-muscular adipose tissue (IMAT), and muscle respectively.
Conclusion
Our proposed method based on machine learning produces accurate tissue quantification and showed an effective use of large information provided by the four contrast images from Dixon MRI.







Similar content being viewed by others
References
Wells JCK, Fewtrell MS (2006) Measuring body composition. Arch Dis Child 91(7):612–617
Do Lee C, Blair SN, Jackson AS (1999) Cardiorespiratory fitness, body composition, and all-cause and cardiovascular disease mortality in men. Am J Clin Nutr 69(3):373–380
Park SW, Goodpaster BH, Strotmeyer ES, de Rekeneire N, Harris TB, Schwartz AV, Tylavsky FA, Newman AB (2006) Decreased muscle strength and quality in older adults with type 2 diabetes. The health, aging, and body composition study. Diabetes 55(6):1813–1818
Collaboration PS (2009) Body-mass index and cause-specific mortality in 900,000 adults: collaborative analyses of 57 prospective studies. Lancet 373(9669):1083–1096
Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, Singh GM, Gutierrez HR, Lu Y, Bahalim AN (2011) National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9· 1 million participants. Lancet 377(9765):557–567
Lim JP, Leung BP, Ding YY, Tay L, Ismail NH, Yeo A, Yew S, Chong MS (2015) Monocyte chemoattractant protein-1: a proinflammatory cytokine elevated in sarcopenic obesity. Clin Interv Aging 10:605–609
Wang C, Bai L (2012) Sarcopenia in the elderly: basic and clinical issues. Geriatr Gerontol Int 12(3):388–396
Addison O, Marcus RL, LaStayo PC, Ryan AS (2014) Intermuscular fat: a review of the consequences and causes. Int J Endocrinol
Ma J (2008) Dixon techniques for water and fat imaging. J Magn Reson Imaging 28(3):543–558
Armao D, Guyon JP, Firat Z, Brown MA, Semelka RC (2006) Accurate quantification of visceral adipose tissue (VAT) using water-saturation MRI and computer segmentation: preliminary results. J Magn Reson Imaging 23(5):736–741
Makrogiannis S, Serai S, Fishbein KW, Schreiber C, Ferrucci L, Spencer RG (2012) Automated quantification of muscle and fat in the thigh from water-, fat-, and nonsuppressed MR images. J Magn Reson Imaging 35(5):1152–1161
Kullberg J, Johansson L, Ahlström H, Courivaud F, Koken P, Eggers H, Börnert P (2009) Automated assessment of whole-body adipose tissue depots from continuously moving bed MRI: a feasibility study. J Magn Reson Imaging 30(1):185–193
Sadananthan SA, Prakash B, Leow MKS, Khoo CM, Chou H, Venkataraman K, Khoo EY, Lee YS, Gluckman PD, Tai ES (2015) Automated segmentation of visceral and subcutaneous (deep and superficial) adipose tissues in normal and overweight men. J Magn Reson Imaging 41(4):924–934
Wald D, Teucher B, Dinkel J, Kaaks R, Delorme S, Boeing H, Seidensaal K, Meinzer HP, Heimann T (2012) Automatic quantification of subcutaneous and visceral adipose tissue from whole-body magnetic resonance images suitable for large cohort studies. J Magn Reson Imaging 36(6):1421–1434
Leinhard OD, Johansson A, Rydell J, Smedby Ö, NystrÖm F, Lundberg P, Borga M (2008) Quantitative abdominal fat estimation using MRI. In: Pattern recognition, 2008. ICPR 2008. 19th International Conference on, 2008. IEEE, pp 1–4
Karlsson A, Rosander J, Romu T, Tallberg J, Grönqvist A, Borga M, Dahlqvist Leinhard O (2015) Automatic and quantitative assessment of regional muscle volume by multi-atlas segmentation using whole-body water–fat MRI. J Magn Reson Imaging 41(6):1558–1569
Wang D, Shi L, Chu WC, Hu M, Tomlinson B, Huang W-H, Wang T, Heng PA, Yeung DK, Ahuja AT (2015) Fully automatic and nonparametric quantification of adipose tissue in fat–water separation MR imaging. Med Biol Eng Comput 53(11):1247–1254
Joshi AA, Hu HH, Leahy RM, Goran MI, Nayak KS (2013) Automatic intra-subject registration-based segmentation of abdominal fat from water–fat MRI. J Magn Reson Imaging 37(2):423–430
Kullberg J, Karlsson AK, Stokland E, Svensson PA, Dahlgren J (2010) Adipose tissue distribution in children: automated quantification using water and fat MRI. J Magn Reson Imaging 32(1):204–210
Valentinitsch A, Karampinos DC, Alizai H, Subburaj K, Kumar D, Link MT, Majumdar S (2013) Automated unsupervised multi-parametric classification of adipose tissue depots in skeletal muscle. J Magn Reson Imaging 37(4):917–927
Tustison NJ, Avants BB, Cook P, Zheng Y, Egan A, Yushkevich P, Gee JC (2010) N4ITK: improved N3 bias correction. IEEE Trans Med Imaging 29(6):1310–1320
Kass M, Witkin A, Terzopoulos D (1988) Snakes: active contour models. Int J Comput Vision 1(4):321–331
Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G (2006) User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 31(3):1116–1128
Huang G-B, Zhu Q-Y, Siew C-K (2006) Extreme learning machine: theory and applications. Neurocomputing 70(1):489–501
Dice LR (1945) Measures of the amount of ecologic association between species. Ecology 26(3):297–302
MATLAB (2010) Version 7.11.1 edn. The MathWorks Inc., Natick, Massachusetts
Conover WJ (1998) Practical nonparametric statistics
StataCorp (2015) Stata statistical software: release 14. StataCorp LP, College Station
Hall LO, Bensaid AM, Clarke LP, Velthuizen RP, Silbiger MS, Bezdek JC (1992) A comparison of neural network and fuzzy clustering techniques in segmenting magnetic resonance images of the brain. IEEE Trans Neural Netw 3(5):672–682
Rajon DA, Jokisch DW, Patton PW, Shah AP, Watchman CJ, Bolch WE (2002) Voxel effects within digital images of trabecular bone and their consequences on chord-length distribution measurements. Phys Med Biol 47(10):1741
Acknowledgments
This study was funded by Lee Foundation Grant 2013 (GERILABS—Longitudinal Assessment of Biomarkers for characterisation of early Sarcopenia and predicting frailty and functional decline in community-dwelling Asian older adults Study).
We would like to acknowledge Assistant Professor Poh Chueh Loo from Nanyang Technological University for his valuable advices in segmentation techniques. We would also like to extend our thanks to Mr. Samuel Neo, Medical Social Worker, Department of Continuing and Community Care, Tan Tock Seng Hospital, for his assistance in recruitment through the various Senior Activity Centres (SAC). We extend our appreciation to the following SACs (Wesley SAC, Care Corner SAC, Xin Yuan Community Service, Potong Pasir Wellness Centre, Tung Ling Community Services (Marine Parade and Bukit Timah), Viriya Community Services-My Centre@Moulmein, House of Joy) and the study participants who have graciously consented to participation in the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Yang, Y.X., Chong, M.S., Tay, L. et al. Automated assessment of thigh composition using machine learning for Dixon magnetic resonance images. Magn Reson Mater Phy 29, 723–731 (2016). https://doi.org/10.1007/s10334-016-0547-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10334-016-0547-2