Abstract
Objective
Myeloma Response Assessment and Diagnosis System recently published provides a framework for the standardised interpretation of DW-WBMRI in response assessment of multiple myeloma (MM) based on expert opinion. However, there is a lack of meta-analysis providing higher-level evidence to support the recommendations. In addition, some disagreement exists in the literature regarding the effect of timing and lesion subtypes on apparent diffusion coefficient (ADC) value changes post-treatment.
Method
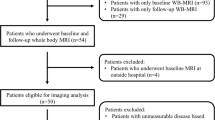
Medline, Cochrane and Embase were searched from inception to 20th July 2021, using terms reflecting multiple myeloma and DW-WBMRI. Using PRISMA reporting guidelines, data were extracted by two investigators. Quality was assessed by the Quality Assessment of Diagnostic Accuracy Studies-2 method.
Results
Of the 74 papers screened, 10 studies were included comprising 259 patients (127 males and 102 females) and 1744 reported lesions. Responders showed a significant absolute ADC change of 0.21×10−3 mm/s2 (95% CI, 0.01–0.41) with little evidence of heterogeneity (Cochran Q, p = 0.12, I2 = 45%) or publication bias (p = 0.737). Non-responders did not show a significant absolute difference in ADC (0.06 ×10−3 mm/s2, 95% CI, −0.07 to 0.19). A percentage ADC increase of 34.78% (95% CI, 10.75–58.81) was observed in responders. Meta-regression showed an inverse trend between ADC increases and time since chemotherapy initiation which did not reach statistical significance (R2 = 20.46, p = 0.282).
Conclusions
This meta-analysis supports the use of the DW-WBMRI as an imaging biomarker for response assessment. More evidence is needed to further characterise ADC changes by lesion subtypes over time.
Key Points
• In multiple myeloma patients who received chemotherapy, responders have a significant absolute increase in ADC values that is not seen in non-responders.
• A 35% increase in ADC from baseline values is found to classify response post-induction chemotherapy which corroborates with expert opinion from the Myeloma Response Assessment and Diagnosis System.
• More evidence is needed to further characterise ADC changes by lesion subtypes over time after induction of therapy.




Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- DW-WBMRI:
-
Diffusion-weighted whole-body magnetic resonance imaging
- IMWG:
-
International Myeloma Working Group
- MET-RADS:
-
Metastasis Reporting and Data System for Prostate Cancer
- MM:
-
Multiple myeloma
- MY-RADS:
-
Myeloma Response Assessment and Diagnosis System
- NICE:
-
National Institute for Health and Care Excellence
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- QUADAS:
-
Quality Assessment of Diagnostic Accuracy Studies
References
Barwick T, Bretsztajn L, Wallitt K et al (2019) Imaging in myeloma with focus on advanced imaging techniques. Br J Radiol 92:20180768
Cancer Research UK (2017) Myeloma incidence statistics. https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/myeloma/incidence. Accessed 20 Aug 2020
Sung H, Ferlay J, Siegel RL et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. https://doi.org/10.3322/caac.21660.Epu
Harry VN, Semple SI, Parkin DE, Gilbert FJ (2010) Use of new imaging techniques to predict tumour response to therapy. Lancet Oncol 11:92–102
Kumar S, Paiva B, Anderson KC et al (2016) International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol 17:e328–e346
Chantry A, Kazmi M, Barrington S et al (2017) Guidelines for the use of imaging in the management of patients with myeloma. Br J Haematol 178:380–393. https://doi.org/10.1111/bjh.14827
The National Institute for Health and Care Excellence (NICE) (2018) Myeloma: diagnosis and management (NG35). https://www.nice.org.uk/guidance/ng35. Accessed 10 Aug 2020
Messiou C, Hillengass J, Delorme S et al (2019) Guidelines for acquisition, interpretation, and reporting of whole-body MRI in myeloma: Myeloma Response Assessment and Diagnosis System (MY-RADS). Radiology 291:5–13. https://doi.org/10.1148/radiol.2019181949
Yao K, Troupis JM (2016) Diffusion-weighted imaging and the skeletal system: a literature review. Clin Radiol 71:1071–1082
Hillengass J, Bäuerle T, Bartl R et al (2011) Diffusion-weighted imaging for non-invasive and quantitative monitoring of bone marrow infiltration in patients with monoclonal plasma cell disease: a comparative study with histology. Br J Haematol 153:721–728. https://doi.org/10.1111/j.1365-2141.2011.08658.x
Dutoit JC, Verstraete KL (2016) MRI in multiple myeloma: a pictorial review of diagnostic and post-treatment findings. Insights Imaging 7:553–569
Fenchel M, Konaktchieva M, Weisel K et al (2010) Response assessment in patients with multiple myeloma during antiangiogenic therapy using arterial spin labeling and diffusion-weighted imaging. A feasibility study. Acad Radiol 17:1326–1333. https://doi.org/10.1016/j.acra.2010.08.002
Horger M, Weisel K, Horger W et al (2011) Whole-body diffusion-weighted MRI with apparent diffusion coefficient mapping for early response monitoring in multiple myeloma: preliminary results. AJR Am J Roentgenol 196:W790–W795. https://doi.org/10.2214/AJR.10.5979
Messiou C, Giles S, Collins DJ et al (2012) Assessing response of myeloma bone disease with diffusion-weighted MRI. Br J Radiol 85:e1198–e1203. https://doi.org/10.1259/bjr/52759767
Giles SL, Messiou C, Collins DJ et al (2014) Whole-body diffusion-weighted MR imaging for assessment of treatment response in myeloma. Radiology 271:785–794. https://doi.org/10.1148/radiol.13131529
Bonaffini PA, Ippolito D, Casiraghi A et al (2015) Apparent diffusion coefficient maps integrated in whole-body MRI examination for the evaluation of tumor response to chemotherapy in patients with multiple myeloma. Acad Radiol 22:1163–1171. https://doi.org/10.1016/j.acra.2015.05.011
Dutoit JC, Claus E, Offner F, Noens L, Delanghe J, Verstraete KL (2016) Combined evaluation of conventional MRI, dynamic contrast-enhanced MRI and diffusion weighted imaging for response evaluation of patients with multiple myeloma. Eur J Radiol 85:373–382. https://doi.org/10.1016/j.ejrad.2015.11.040
Latifoltojar A, Hall-Craggs M, Bainbridge A et al (2017) Whole-body MRI quantitative biomarkers are associated significantly with treatment response in patients with newly diagnosed symptomatic multiple myeloma following bortezomib induction. Eur Radiol 27:5325–5336. https://doi.org/10.1007/s00330-017-4907-8
Lacognata C, Crimì F, Guolo A et al (2017) Diffusion-weighted whole-body MRI for evaluation of early response in multiple myeloma. Clin Radiol 72:850–857. https://doi.org/10.1016/j.crad.2017.05.004
Wu C, Huang J, Bin XW et al (2018) Discriminating depth of response to therapy in multiple myeloma using whole-body diffusion-weighted MRI with apparent diffusion coefficient: preliminary results from a single-center study. Acad Radiol 25:904–914. https://doi.org/10.1016/j.acra.2017.12.008
Zhang Y, Xiong X, Fu Z et al (2019) Whole-body diffusion-weighted MRI for evaluation of response in multiple myeloma patients following bortezomib-based therapy: a large single-center cohort study. Eur J Radiol 120:108695. https://doi.org/10.1016/j.ejrad.2019.108695
Takasu M, Kondo S, Akiyama Y et al (2020) Assessment of early treatment response on MRI in multiple myeloma: comparative study of whole-body diffusion-weighted and lumbar spinal MRI. PLoS One 15:e0229607. https://doi.org/10.1371/journal.pone.0229607
Park HY, Kim KW, Yoon MA et al (2020) Role of whole-body MRI for treatment response assessment in multiple myeloma: comparison between clinical response and imaging response. Cancer Imaging 20:14. https://doi.org/10.1186/s40644-020-0293-6
Whiting PF, Rutjes AWS, Westwood ME et al (2011) QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 155:529–536. https://doi.org/10.7326/0003-4819-155-8-201110180-00009
Sheldon J, Wheeler RD, Powles R (2017) Electrophoretic patterns post daratumumab. Ann Clin Biochem 55:299–301. https://doi.org/10.1177/0004563217710489
Udd KA, Spektor TM, Berenson JR (2017) Monitoring multiple myeloma. Clin Adv Hematol Oncol 15:951–961
Caers J, Laurent G, Martin Kortüm K et al (2018) European myeloma network recommendations on tools for the diagnosis and monitoring of multiple myeloma: what to use and when. Haematologica 103:1772–1784. https://doi.org/10.3324/haematol.2018.189159
Higgins JPT, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. Br Med J 327:557–560
Egger M, Smith GD, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. Br Med J 315:629–634. https://doi.org/10.1136/bmj.315.7109.629
Peters JL, Sutton AJ, Jones DR et al (2008) Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol 61:991–996. https://doi.org/10.1016/j.jclinepi.2007.11.010
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Padhani AR, Lecouvet FE, Tunariu N et al (2017) METastasis reporting and data system for prostate cancer: practical guidelines for acquisition, interpretation, and reporting of whole-body magnetic resonance imaging-based evaluations of multiorgan involvement in advanced prostate cancer. Eur Urol 71:81–92. https://doi.org/10.1016/j.eururo.2016.05.033
Messiou C, Collins DJ, Morgan VA, Desouza NM (2011) Optimising diffusion weighted MRI for imaging metastatic and myeloma bone disease and assessing reproducibility. Eur Radiol 21:1713–1718. https://doi.org/10.1007/s00330-011-2116-4
Koh D-M, Blackledge M, Collins DJ et al (2009) Reproducibility and changes in the apparent diffusion coefficients of solid tumours treated with combretastatin A4 phosphate and bevacizumab in a two-centre phase I clinical trial. Eur Radiol 19:2728–2738. https://doi.org/10.1007/s00330-009-1469-4
Kloth JK, Hillengass J, Listl K et al (2014) Appearance of monoclonal plasma cell diseases in whole-body magnetic resonance imaging and correlation with parameters of disease activity. Int J Cancer 135:2380–2386. https://doi.org/10.1002/ijc.28877
Koutoulidis V, Fontara S, Terpos E et al (2017) Quantitative diffusion-weighted imaging of the bone marrow: an adjunct tool for the diagnosis of a diffuse MR imaging pattern in patients with multiple myeloma. Radiology 282:484–493. https://doi.org/10.1148/radiol.2016160363
Anderson KC (2017) Should minimal residual disease negativity be the end point of myeloma therapy? Blood Adv 1:517–521. https://doi.org/10.1182/bloodadvances.2016000117
Rasche L, Alapat D, Kumar M et al (2019) Combination of flow cytometry and functional imaging for monitoring of residual disease in myeloma. Leukemia 33:1713–1722. https://doi.org/10.1038/s41375-018-0329-0
Zamagni E, Tacchetti P, Barbato S, Cavo M (2020) Role of imaging in the evaluation of minimal residual disease in multiple myeloma patients. J Clin Med 9:3519. https://doi.org/10.3390/jcm9113519
Lokhorst HM, Plesner T, Laubach JP et al (2015) Targeting CD38 with daratumumab monotherapy in multiple myeloma. N Engl J Med 373:1207–1219. https://doi.org/10.1056/NEJMoa1506348
Raje N, Berdeja J, Lin Y et al (2019) Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N Engl J Med 380:1726–1737. https://doi.org/10.1056/NEJMoa1817226
Yan Z, Cao J, Cheng H et al (2019) A combination of humanised anti-CD19 and anti-BCMA CAR T cells in patients with relapsed or refractory multiple myeloma: a single-arm, phase 2 trial. Lancet Haematol 6:e521–e529. https://doi.org/10.1016/S2352-3026(19)30115-2
Mikkilineni L, Kochenderfer JN (2021) CAR T cell therapies for patients with multiple myeloma. Nat Rev Clin Oncol 18:71–84. https://doi.org/10.1038/s41571-020-0427-6
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Sola Adeleke.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise in meta-analysis.
Informed consent
Written informed consent was not required for this study because this study is a meta-analysis of previously published literature.
Ethical approval
Institutional Review Board approval was not required because this study is a meta-analysis of previously published literature.
Study subjects or cohorts overlap
All study subjects or cohorts have been previously published.
Methodology
-
Mix of prospective and retrospective
-
prognostic study
-
multicenter meta-analysis
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 14.4 kb)
Rights and permissions
About this article
Cite this article
Wang, K., Lee, E., Kenis, S. et al. Application of diffusion-weighted whole-body MRI for response monitoring in multiple myeloma after chemotherapy: a systematic review and meta-analysis. Eur Radiol 32, 2135–2148 (2022). https://doi.org/10.1007/s00330-021-08311-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-021-08311-z