Abstract
Objective
To evaluate the role of diffusion-weighted imaging (DWI) in the initial diagnosis, staging, and assessment of treatment response in patients with multiple myeloma (MM).
Materials and methods
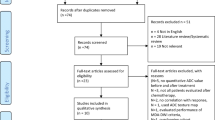
A systematic literature review was conducted in PubMed, the Cochrane Library, EMBASE, Scopus, and Web of Science databases. The primary endpoints were defined as the diagnostic performance of DWI for disease detection, staging of MM, and assessing response to treatment in these patients.
Results
Of 5881 initially reviewed publications, 33 were included in the final qualitative and quantitative meta-analysis. The diagnostic performance of DWI in the detection of patients with MM revealed pooled sensitivity and specificity of 86% (95% CI: 84–89) and 63% (95% CI: 56–70), respectively, with a diagnostic odds ratio (OR) of 14.98 (95% CI: 4.24–52.91). The pooled risk difference of 0.19 (95% CI: − 0.04–0.42) was reported in favor of upstaging with DWI compared to conventional MRI (P value = 0.1). Treatment response evaluation and ADCmean value changes across different studies showed sensitivity and specificity of approximately 78% (95% CI: 72–83) and 73% (95% CI: 61–83), respectively, with a diagnostic OR of 7.21 in distinguishing responders from non-responders.
Conclusions
DWI is not only a promising tool for the diagnosis of MM, but it is also useful in the initial staging and re-staging of the disease and treatment response assessment. This can aid clinicians with earlier initiation or change in treatment strategy, which could have prognostic significance for patients.












Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- IMWG:
-
International Myeloma Working Group
- MGUS:
-
Monoclonal gammopathy of undetermined significance
- MM:
-
Multiple myeloma
- MRI:
-
Magnetic resonance imaging
- MY-RADS:
-
Myeloma Response Assessment and Diagnosis System
- NLR:
-
Negative likelihood ratio
- PLR:
-
Positive likelihood ratio
- ROC:
-
Receiver operating characteristic
- SMM:
-
Smoldering multiple myeloma
- SNR:
-
Signal-to-noise ratio
- WBLD-CT:
-
Whole-body low-dose computed tomography
- WB-MRI:
-
Whole-body MRI
- 18F-FDG PET/CT:
-
Fluorine-18 fluorodeoxyglucose positron emission tomography with computed tomography
References
Kyle RA, Durie BGM, Rajkumar SV, Landgren O, Blade J, Merlini G, et al. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia. 2010;24:1121–7.
Smith D, Yong K. Multiple myeloma. BMJ. 2013;346: f3863.
Smith A, Wisloff F, Samson D. UK Myeloma Forum, Nordic Myeloma Study Group, British Committee for Standards in Haematology. Guidelines on the diagnosis and management of multiple myeloma 2005. Br J Haematol. 2006;132:410–51.
Engelhardt M, Kleber M, Frydrychowicz A, Pache G, Schmitt-Gräff A, Wäsch R, et al. Superiority of magnetic resonance imaging over conventional radiographs in multiple myeloma. Anticancer Res. 2009;29:4745–50.
Petralia G, Padhani AR, Pricolo P, Zugni F, Martinetti M, Summers PE, et al. Whole-body magnetic resonance imaging (WB-MRI) in oncology: recommendations and key uses. Radiol Med. 2019;124:218–33.
Jacobs MA, Pan L, Macura KJ. Whole-body diffusion-weighted and proton imaging: a review of this emerging technology for monitoring metastatic cancer. Semin Roentgenol. 2009;44:111–22.
Ghanem N, Lohrmann C, Engelhardt M, Pache G, Uhl M, Saueressig U, et al. Whole-body MRI in the detection of bone marrow infiltration in patients with plasma cell neoplasms in comparison to the radiological skeletal survey. Eur Radiol. 2006;16:1005–14.
Horger M, Weisel K, Horger W, Mroue A, Fenchel M, Lichy M. Whole-body diffusion-weighted MRI with apparent diffusion coefficient mapping for early response monitoring in multiple myeloma: preliminary results. AJR Am J Roentgenol. 2011;196:W790-795.
Latifoltojar A, Hall-Craggs M, Bainbridge A, Rabin N, Popat R, Rismani A, et al. Whole-body MRI quantitative biomarkers are associated significantly with treatment response in patients with newly diagnosed symptomatic multiple myeloma following bortezomib induction. Eur Radiol. 2017;27:5325–36.
Latifoltojar A, Yong KL, Hall-Craggs M, Rabin N, Popat R, Bainbridge A, et al. Whole body (WB) MRI in newly diagnosed multiple myeloma (MM): fat fraction changes at 8 weeks predict response to induction with bortezomib regimens. Blood. 2015;126:1850–1850.
Giles SL, Messiou C, Collins DJ, Morgan VA, Simpkin CJ, West S, et al. Whole-body diffusion-weighted MR imaging for assessment of treatment response in myeloma. Radiology. 2014;271:785–94.
Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic re MA, Palumbo A, et al., International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538–48.
International Myeloma Working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121:749–57.
Sachpekidis C, Mosebach J, Freitag MT, Wilhelm T, Mai EK, Goldschmidt H, et al. Application of 18F-FDG PET and diffusion weighted imaging (DWI) in multiple myeloma: comparison of functional imaging modalities. Am J Nucl Med Mol Imaging. 2015;5:479–92.
Singh S, Pilavachi E, Dudek A, Bray TJP, Latifoltojar A, Rajesparan K, et al. Whole body MRI in multiple myeloma: optimising image acquisition and read times. PLoS ONE. 2020;15: e0228424.
Withofs N, Cousin F, Tancredi T, Simoni P, Prijck BD, Hafraoui K, et al. Comparison of combined whole-body [18F]NaF and [18F]FDG PET/CT versus MRI for the detection of myeloma lesions. The Journal of Nuclear Medicine. 2016;57:606–606.
Durie BGM, Harousseau J-L, Miguel JS, Bladé J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–73.
Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17:e328–46.
Terpos E, Koutoulidis V, Fontara S, Zagouri F, Matsaridis D, Christoulas D, et al. Diffusion-weighted magnetic resonance imaging (DW-MRI) is able to distinguish diffuse from normal MRI pattern of marrow involvement in patients with multiple myeloma. Blood. 2014;124:3461–3461.
Koutoulidis V, Fontara S, Terpos E, Zagouri F, Matsaridis D, Christoulas D, et al. Quantitative diffusion-weighted imaging of the bone marrow: an adjunct tool for the diagnosis of a diffuse MR imaging pattern in patients with multiple myeloma. Radiology. 2017;282:484–93.
Narquin S, Ingrand P, Azais I, Delwail V, Vialle R, Boucebci S, et al. Comparison of whole-body diffusion MRI and conventional radiological assessment in the staging of myeloma. Diagn Interv Imaging. 2013;94:629–36.
Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–73.
Durie BG, Salmon SE. A clinical staging system for multiple myeloma. Correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer. 1975;36:842–54.
Wang PF, Li YC, Xu YX, Wang XM, Guo LF, Fu C. [Role of whole-body diffusion weighted imaging (WB-DWI) in the diagnosis and monitoring of newly diagnosed multiple myeloma]. Zhonghua xue ye xue za zhi = Zhonghua xueyexue zazhi. 2017;38 2:129–33.
Klein B, Tarte K, Jourdan M, Mathouk K, Moreaux J, Jourdan E, et al. Survival and proliferation factors of normal and malignant plasma cells. Int J Hematol. 2003;78:106–13.
Weininger M, Lauterbach B, Knop S, Pabst T, Kenn W, Hahn D, et al. Whole-body MRI of multiple myeloma: comparison of different MRI sequences in assessment of different growth patterns. Eur J Radiol. 2009;69:339–45.
Park HY, Kim KW, Yoon MA, Lee MH, Chae EJ, Lee JH, et al. Role of whole-body MRI for treatment response assessment in multiple myeloma: comparison between clinical response and imaging response. Cancer Imaging. 2020;20:14.
Takasu M, Kondo S, Akiyama Y, Takahashi Y, Maeda S, Baba Y, et al. Assessment of early treatment response on MRI in multiple myeloma: comparative study of whole-body diffusion-weighted and lumbar spinal MRI. PLoS ONE. 2020;15: e0229607.
Pawlyn C, Fowkes L, Otero S, Jones JR, Boyd KD, Davies FE, et al. Whole-body diffusion-weighted MRI: a new gold standard for assessing disease burden in patients with multiple myeloma? Leukemia. 2016;30:1446–8.
Decaux O, et al. Evaluation of whole-body diffusion-weighted magnetic resonance imaging for diagnosis and monitoring of multiple myeloma, Haematologica. 2011;96:S65–6.
Brilleta É, Decauxc O, Bernardc A-M, Devillersc A, Chapellec T, Bertaud V, et al. Comparison between whole-body diffusion MRI and 18FDG PET/CT in the diagnosis of symptomatic myeloma. Medecine Nucleaire. 2012;36:313–9.
Giles SL, deSouza NM, Collins DJ, Morgan VA, West S, Davies FE, et al. Assessing myeloma bone disease with whole-body diffusion-weighted imaging: comparison with x-ray skeletal survey by region and relationship with laboratory estimates of disease burden. Clin Radiol. 2015;70:614–21.
Barwick T, Orton M, Koh DM, Kaiser M, Rockall A, Tunariu N, et al. Repeatability and reproducibility of apparent diffusion coefficient and fat fraction measurement of focal myeloma lesions on whole body magnetic resonance imaging. BJR The British Institute of Radiology. 2021;94:20200682.
Mosebach J, Sachpekidis C, Fard N, Wilhelm T, Wilhelm M, Hillengass J, et al. Characterization of multiple myeloma osseous lesions and diffuse infiltration pattern by 18F-FDG-PET/CT, static MRI and diffusion-weighted MR Imaging (DWI-MRI): a comparative multimodality imaging study. Cancer Imaging. 2014;14:P33–P33.
Berno T, et al. Whole-body diffusion-weighted MRI and 18F-FDG PET-CT in the assessment of bone disease in multiple myeloma. Clinical Lymphoma, Myeloma and Leukemia. 2013;13:S84.
Rasche L, Angtuaco E, McDonald JE, Buros A, Stein C, Pawlyn C, et al. Low expression of hexokinase-2 is associated with false-negative FDG–positron emission tomography in multiple myeloma. Blood. 2017;130:30–4.
Chenevert TL, Sundgren PC, Ross BD. Diffusion imaging: insight to cell status and cytoarchitecture. Neuroimaging Clinics Elsevier. 2006;16:619–32.
Hillengass J, Bäuerle T, Bartl R, Andrulis M, McClanahan F, Laun FB, et al. Diffusion-weighted imaging for non-invasive and quantitative monitoring of bone marrow infiltration in patients with monoclonal plasma cell disease: a comparative study with histology. Br J Haematol. 2011;153:721–8.
Lemke A, et al. Differentiation of pancreas carcinoma from healthy pancreatic tissue using multiple b-values: comparison of apparent diffusion coefficient and intravoxel incoherent motion derived parameters. Invest Radiol. 2009;44(12):769–75. (https://pubmed.ncbi.nlm.nih.gov/19838121/)
Luciani A, Vignaud A, Cavet M, Nhieu JTV, Mallat A, Ruel L, et al. Liver cirrhosis: intravoxel incoherent motion MR imaging – pilot study. Radiology. 2008;249:891–9.
Le Bihan D. Intravoxel incoherent motion imaging using steady-state free precession. Magn Reson Med. 1988;7:346–51 (John Wiley & Sons, Ltd).
Sezer O, Niemöller K, Jakob C, Zavrski I, Heider U, Eucker J, et al. Relationship between bone marrow angiogenesis and plasma cell infiltration and serum beta2-microglobulin levels in patients with multiple myeloma. Annals of hematology. 2001;80(10):598–601.
Winfield JM, Poillucci G, Blackledge MD, Collins DJ, Shah V, Tunariu N, et al. Apparent diffusion coefficient of vertebral haemangiomas allows differentiation from malignant focal deposits in whole-body diffusion-weighted MRI. Eur Radiol. 2018;28:1687–91.
Messiou C, Giles S, Collins DJ, West S, Davies FE, Morgan GJ, et al. Assessing response of myeloma bone disease with diffusion-weighted MRI. Br J Radiol. 2012;85:e1198–203.
Lavdas I, Rockall AG, Castelli F, Sandhu RS, Papadaki A, Honeyfield L, et al. Apparent diffusion coefficient of normal abdominal organs and bone marrow from whole-body DWI at 1.5 T: the effect of sex and age. Am J Roentgenol Am Roentgen Ray Soc. 2015;205:242–50.
Herneth AM, Friedrich K, Weidekamm C, Schibany N, Krestan C, Czerny C, et al. Diffusion weighted imaging of bone marrow pathologies. Eur J Radiol. 2005;55:74–83.
Dutoit JC, Vanderkerken MA, Anthonissen J, Dochy F, Verstraete KL. The diagnostic value of SE MRI and DWI of the spine in patients with monoclonal gammopathy of undetermined significance, smouldering myeloma and multiple myeloma. Eur Radiol. 2014;24:2754–65.
Messiou C, Collins DJ, Morgan VA, Desouza NM. Optimising diffusion weighted MRI for imaging metastatic and myeloma bone disease and assessing reproducibility. Eur Radiol. 2011;21:1713–8.
Hernandez JM, Montesinos O, Mateo AG, Queizán JA, del Peral GS, Olivier C, et al. Usefulness of whole-body diffusion-weighted MRI (WB-MRI) with apparent diffusion coefficient (ADC) in the differentiation of monoclonal gammopathies. Clin Lymphoma, Myeloma and Leukemia. 2015;15:e90-1 (Elsevier).
Burns R, et al. Whole-body PET/MRI for multiple myeloma staging: Impact of 18F-FDG uptake and MR sequence design on focal lesion and bone marrow infiltration assessment. Eur J Nucl Med Mol Imaging. 2018;45:S235.
Squillaci E, Bolacchi F, Altobelli S, Franceschini L, Bergamini A, Cantonetti M, et al. Pre-treatment staging of multiple myeloma patients: comparison of whole-body diffusion weighted imaging with whole-body T1-weighted contrast-enhanced imaging. Acta Radiol SAGE Publications. 2015;56:733–8.
Auger S, Exbrayat C, Decoux E, Aufort S, Plassot C, Donadio D, et al. Contribution of diffusion and ADC sequences in whole body MRI for myeloma staging. Haematologica. Ferrata Storti Foundation via Guiseppe Belli 4, 27100 Pavia, Italy; 2013. p. 599–599.
Bourillon C, Rahmouni A, Lin C, Belhadj K, Beaussart P, Vignaud A, et al. Intravoxel incoherent motion diffusion-weighted imaging of multiple myeloma lesions: correlation with whole-body dynamic contrast agent–enhanced MR imaging. Radiol Radiol Soc North Am. 2015;277:773–83.
Shah R, Stieltjes B, Andrulis M, Pfeiffer R, Sumkauskaite M, Delorme S, et al. Intravoxel incoherent motion imaging for assessment of bone marrow infiltration of monoclonal plasma cell diseases. Ann Hematol. 2013;92:1553–7 (Springer).
Padhani A. Guidelines for acquisition, interpretation, and reporting of whole-body MRI in myeloma: myeloma response assessment and diagnosis system. Radiology. 2019;291:5–13.
Sommer G, Klarhöfer M, Lenz C, Scheffler K, Bongartz G, Winter L. Signal characteristics of focal bone marrow lesions in patients with multiple myeloma using whole body T1w-TSE, T2w-STIR and diffusion-weighted imaging with background suppression. Eur Radiol. 2011;21:857–62 (Springer).
Wu C, Huang J, Xu W-B, Guan Y-J, Ling H-W, Mi J-Q, et al. Discriminating depth of response to therapy in multiple myeloma using whole-body diffusion-weighted MRI with apparent diffusion coefficient: preliminary results from a single-center study. Acad Radiol. 2018;25:904–14 (Elsevier).
Huang W, Yang M, Sui W, Deng S, Liu W, Ji X, et al. The correlation between findings of whole-body diffusion weighted imaging and clinical result in patients with multiple myeloma. Zhonghua Yi Xue Za Zhi. 2019;99:664–8.
Lacognata C, Crimì F, Guolo A, Varin C, De March E, Vio S, et al. Diffusion-weighted whole-body MRI for evaluation of early response in multiple myeloma. Clin Radiol. 2017;72:850–7 (Elsevier).
Horger M, Weisel K, Bares R, Ernemann U, Claussen C, Lichy M, et al. Modern imaging techniques during therapy in patients with multiple myeloma. Acta radiologica. SAGE Publications Sage UK: London, England; 2011;52:881–8.
Bonaffini PA, Ippolito D, Casiraghi A, Besostri V, Franzesi CT, Sironi S. Apparent diffusion coefficient maps integrated in whole-body MRI examination for the evaluation of tumor response to chemotherapy in patients with multiple myeloma. Acad Radiol. 2015;22:1163–71 (Elsevier).
Wang M, Delasalle K, Feng L, Thomas S, Giralt S, Qazilbash M, et al. CR represents an early index of potential long survival in multiple myeloma. Bone Marrow Transplant. 2010;45:498–504 (Nature Publishing Group).
Moreau P, Attal M, Facon T. Frontline therapy of multiple myeloma. Blood. 2015;125:3076–84.
Rosiñol L, Oriol A, Teruel AI, Hernández D, López-Jiménez J, de la Rubia J, et al. Superiority of bortezomib, thalidomide, and dexamethasone (VTD) as induction pretransplantation therapy in multiple myeloma: a randomized phase 3 PETHEMA/GEM study. Blood, The Journal of the American Society of Hematology. American Society of Hematology Washington, DC; 2012;120:1589–96.
Rasche L, Schinke CD, Alapat D, Gershner G, Johnson SK, Thanendrarajan S, et al. Functional imaging detects residual disease in MRD-negative multiple myeloma patients who subsequently relapse. Blood. 2017;130:4510 (Elsevier).
Zamagni E, Tacchetti P, Terragna C, Cavo M. Multiple myeloma: disease response assessment. Expert Rev Hematol. 2016;9:831–7 (Taylor & Francis).
Stetler-Stevenson M, Paiva B, Stoolman L, Lin P, Jorgensen JL, Orfao A, et al. Consensus guidelines for myeloma minimal residual disease sample staining and data acquisition. Cytometry Part B Clin Cytometry. 2016;90:26–30 (Wiley Online Library).
Spina F, Potepan P, Trecate G, Montin E, Montefusco V, Laffranchi A, et al. Results of a prospective study comparing whole-body diffusion-weighted magnetic resonance imaging with skeletal X-ray and magnetic resonance of the spine for assessing bone disease in multiple myeloma. Blood. 2012;120:2913 (Elsevier).
Fernández-Poveda E, Cabañas V, Moreno MJ, Blanquer Blanquer M, Moraleda JM. Prognostic value of diffusion-weighted magnetic resonance imaging in newly diagnosed multiple myeloma patients treated with up-front autologous transplantation. Blood. 2019;134:3146.
Latifoltojar A, Hall-Craggs M, Rabin N, Popat R, Bainbridge A, Dikaios N, et al. Whole body magnetic resonance imaging in newly diagnosed multiple myeloma: early changes in lesional signal fat fraction predict disease response. Br J Haematol. 2017;176:222–33 (Wiley Online Library).
Padhani AR, Koh D-M, Collins DJ. Whole-body diffusion-weighted MR imaging in cancer: current status and research directions. Radiology. 2011;261:700–18 (Radiological Society of North America, Inc.).
Lecouvet F, et al. MRI for response assessment in metastatic bone disease. Eur Radiol. 2013;23(7):1986–97. (https://pubmed.ncbi.nlm.nih.gov/23455764/).
Lecouvet FE, Vekemans M-C, Van Den Berghe T, Verstraete K, Kirchgesner T, Acid S, et al. Imaging of treatment response and minimal residual disease in multiple myeloma: state of the art WB-MRI and PET/CT. Skelet Radiol. Springer; 2021;1–22. ( https://pubmed.ncbi.nlm.nih.gov/34363522/)
Mahale A, Maruvaneni S, Kumar A, Ullal S, Fernandes M. Role of Diffusion Weighted imaging in differentiating benign from pathological vertebral collapse using ADC values. J Clin Diagn Res 2019;13.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
For this type of study, consent for publication is not required.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
• The development of functional MRI sequences such as diffusion-weighted imaging (DWI) has made a functional assessment of lesions feasible.
• Diffusion-weighted imaging (DWI) has a role in the diagnosis, staging, and treatment response of multiple myeloma.
• DWI can quantitatively assess tissue cellularity by detecting free water molecule movements depicted on apparent diffusion coefficient (ADC) maps.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Torkian, P., Mansoori, B., Hillengass, J. et al. Diffusion-weighted imaging (DWI) in diagnosis, staging, and treatment response assessment of multiple myeloma: a systematic review and meta-analysis. Skeletal Radiol 52, 565–583 (2023). https://doi.org/10.1007/s00256-022-04119-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-022-04119-0